Page 27 - Monitoring Prostate Cancer to Guide Treatment Decision-Making
P. 27
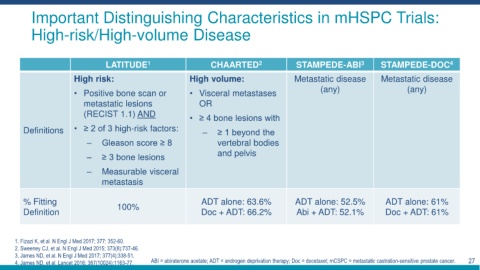
Important Distinguishing Characteristics in mHSPC Trials:
High-risk/High-volume Disease
LATITUDE 1 CHAARTED 2 STAMPEDE-ABI 3 STAMPEDE-DOC 4
High risk: High volume: Metastatic disease Metastatic disease
• Positive bone scan or • Visceral metastases (any) (any)
metastatic lesions OR
(RECIST 1.1) AND
• ≥ 4 bone lesions with
Definitions • ≥ 2 of 3 high-risk factors: ‒ ≥ 1 beyond the
‒ Gleason score ≥ 8 vertebral bodies
‒ ≥ 3 bone lesions and pelvis
‒ Measurable visceral
metastasis
% Fitting 100% ADT alone: 63.6% ADT alone: 52.5% ADT alone: 61%
Definition Doc + ADT: 66.2% Abi + ADT: 52.1% Doc + ADT: 61%
1. Fizazi K, et al. N Engl J Med 2017; 377: 352-60.
2. Sweeney CJ, et al. N Engl J Med 2015; 373(8):737-46.
3. James ND, et al. N Engl J Med 2017; 377(4):338-51.
4. James ND, et al. Lancet 2016; 387(10024):1163-77. ABI = abiraterone acetate; ADT = androgen deprivation therapy; Doc = docetaxel; mCSPC = metastatic castration-sensitive prostate cancer. 27