Page 1 - nmCRPC Clinical Support Tool-MARCH2021
P. 1
Treatment of non-metastatic CRPC
THE AR-TARGETED THERAPIES
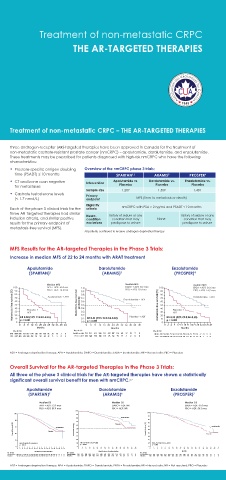
Treatment of non-metastatic CRPC – THE AR-TARGETED THERAPIES
Three androgen-receptor (AR)-targeted therapies have been approved in Canada for the treatment of
non-metastatic castrate-resistant prostate cancer (nmCRPC) – apalutamide, darolutamide, and enzalutamide.
These treatments may be prescribed for patients diagnosed with high-risk nmCRPC who have the following
characteristics:
• Prostate-specific antigen doubling Overview of the nmCRPC phase 3 trials:
time (PSADT) ≤ 10 months SPARTAN 1,2 ARAMIS 3 PROSPER 4
• CT and bone scan negative Intervention Apalutamide vs. Darolutamide vs. Enzalutamide vs.
for metastases Placebo Placebo Placebo
Sample size 1,207 1,509 1,401
• Castrate testosterone levels Primary
(< 1.7 nmol/L) endpoint MFS (time to metastasis or death)
Eligibility
Each of the phase 3 clinical trials for the criteria nmCRPC with PSA > 2 ng/mL and PSADT ≤ 10 months
three AR-targeted therapies had similar Neuro- History of seizure or any History of seizure or any
inclusion criteria, and similar positive condition condition that may None condition that may
results for the primary endpoint of exclusions predispose to seizure predispose to seizure
metastasis-free survival (MFS).
All patients continued to receive androgen-deprivation therapy
MFS Results for the AR-targeted Therapies in the Phase 3 Trials:
nmCRPC Phase 3 Trials: Primary Endpoint – MFSnmCRPC Phase 3 Trials: Primary Endpoint – MFS
nmCRPC Phase 3 Trials: Primary Endpoint – MFS
Increase in median MFS of 22 to 24 months with ARAT treatment
Apalutamide Darolutamide Enzalutamide
Darolutamide
Apalutamide
Apalutamide Enzalutamide Enzalutamide 3 Darolutamide 4 Darolutamide
Apalutamide
Enzalutamide
(SPARTAN)
(ARAMIS)
(PROSPER)
2
(ARAMIS)
(SPARTAN) 2 (PROSPER) 3 2 (PROSPER) 3 4 2 (ARAMIS) 4 3 (ARAMIS) 4
(SPARTAN)
(SPARTAN)
(PROSPER)
Median MFS
Median MFS Median MFS Median MFS Median MFS Median MFS Median MFS
Median MFS
Median MFS
DARO + ADT: 40.4 mo
100 APA + ADT: 40.5 mo 100 APA + ADT: 40.5 mo 100 100 ENZA + ADT: 36.6 mo 1.0 100 DARO + ADT: 40.4 mo 1.0 DARO + ADT: 40.4 mo
APA + ADT: 40.5 mo
100
ENZA + ADT: 36.6 mo
ENZA + ADT: 36.6 mo
PBO + ADT: 18.4 mo
PBO + ADT:
PBO + ADT: 16.2 mo
PBO + ADT: 18.4 mo
PBO + ADT: 18.4 mo
PBO + ADT: 16.2 mo
PBO + ADT: 14.7 mos
PBO + ADT: 14.7 mos 16.2 mo
Metastasis-free survival
PBO + ADT: 14.7 mos
Metastasis-free survival (%) 70 Placebo + Apalutamide + ADT Metastasis-free survival (%) Metastasis-free survival (%) 70 70 Placebo + Apalutamide + ADTT Metastasis-free survival Metastasis-free survival (%) 1.0 Metastasis-free survival (%) 70 ADT Enzalutamide + ADTmide + ADT 0.7 Metastasis-free survival (%) 70 Placebo + Darolutamide + ADT Metastasis-free survival probability 0.7 Darolutamide + ADT
90
90 90
90
0.9
0.9
90
0.9
90
80 80
80
80
0.8
80
80
0.8
0.8
Enzalutamide + AD
Apaluta
Enzalutamide + ADT
probability
0.7
probability
70
Darolutamide + ADT
60
0.6
60
60 60
0.6
60
60
0.6
0.5
50
50 50
50
0.5
50
0.5
50
0.4
40
40 40
0.4
0.4
40
40
40
Placebo + Placebo +
Placebo +
30
0.3
0.3
0.3
30 30
30
30
30
ADT
ADT
ADT
ADT
ADT
20
0.2
20
20
20
HR 0.29 (95% CI 0.24–0.35)0.23–0.35) Placebo + ADT
HR 0.28 (95% Cl
HR 0.29 (95% CI 0.24–0.35)
HR 0.29 (95% CI 0.24–0.35)
0.1
10
0.1
10 10
10
10
0.1
10
p < 0.001
p < 0.001
p < 0.001
p < 0.001
p < 0.001 p < 0.001
p < 0.001
p < 0.001
p < 0.001
0.0
0 HR 0.28 (95% Cl 0.23–0.35) 20 20 HR 0.28 (95% Cl 0.23–0.35) 0.0 HR 0.41 (95% CI 0.34–0.50) 0.2 HR 0.41 (95% CI 0.34–0.50) Placebo + ADT 0.2 HR 0.41 (95% CI 0.34–0.50) Placebo + ADT
0 0
0
0.0
0
0
40
30 33 36 39
0 4 8 12 16 20 24 28 32 36 40 44 0 30 4 6 9 12 15 18 21 28 32 36 40 44 42 0 0 3 6 9 12 15 18 8 12 16 20 240 12 16 20 24 28 32 36 28 3244 48 0 4 8 12 16 20 24 28 32 36 27 40 44 48 39 42 0 4 8 12 16 20 24 28 32 36 40 44 48
8 12 16 20 24 24 27
4
8
30 33 36
3 6 9 12 15 18
21 24
0
30 33 36 39 42 36 40 44
21 24 27
4
Months Months Months Months Months Months
Months
Months
Months
No. at risk No. at risk No. at risk No. at risk No. at risk No. at risk No. at risk
No. at risk
No. at risk
ENZA + ADT 933 865 759 637 528 431 418 328 237 159 87 77 31 96 183768116189262377506
APA + ADT 806 713 652 514 398 282 180 96 36 16 3 0 ENZA + ADT 933 865 759 637 528 431 418 328 237 36 1631 34 0 0 DARO + ADT 955 817 675 4 0 36 2 16 0 DARO + ADT 955 817 675 506 865 759 262 528 189 431 418 68 237 37 159 18 87 77 31 0 4 0 DARO + ADT 955 817 675 506 377 262 189 116 68 37 18 2 0
2
APA + ADT 806 713 652 514 398 282 180 96 159 87 77
0
3
APA + ADT 806 713 652 514 398 282 180
116 328
377 637
ENZA + ADT 933
PBO + ADT 401 291 220 1535075117180
0
PBO + ADT 401 291 220 153 91 58 34 13 5 1 0 0 PBO + ADT 468 420 296 212 157 91 58 34 13 5 1 5 01 0 0 PBO + ADT 554 368 275 91 29 58 12 5 13 04 1 0 5 0 1 0 0 PBO + ADT 554 368 275 468 420 296 212 157 105 98 64 49 31 16 11 0 5 0 1 0 PBO + ADT 554 368 275 180 117 75 50 29 12 4 0 0 0
PBO + ADT 468 420 296 212 157 105 98 64 49 31 16 11 34
117
0
180
50
4
75
29
12
PBO + ADT 401 291 220 153 105 98 64 49 31 16 11
PBO + ADT
ADT = Androgen deprivation therapy; APA = Apalutamide; DARO = Darolutamide; ENZA = Enzalutamide; HR = Hazard ratio; PBO = Placebo
ADT = Androgen deprivation therapy; APA = Apalutamid
ADT = Androgen deprivation therapy; APA = Apalutamide; ADT = Androgen deprivation therapy; APA = Apalutamide; 1. Smith MR, et al. N Engl J Med 2018;378:1408; 1. Smith MR, et al. N Engl J Med 2018;378:1408; e; 1. Smith MR, et al. N Engl J Med 2018;378:1408;
DARO = Darolutamide; ENZA = Enzalutamide; DARO = Darolutamide; ENZA = Enzalutamide; Engl J Med 2018;378:2465; 3. Fizazi K, et al. N Engl J Med 2019;380:1235
DARO = Darolutamide; ENZA = Enzalutamide;
2. Hussain M, et al. N
2. Hussain M, et al. N Engl J Med 2018;378:2465; 3. Fizazi K, et al. N Engl J Med 2019;380:12352. Hussain M, et al. N Engl J Med 2018;378:2465; 3. Fizazi K, et al. N Engl J Med 2019;380:1235
HR = Hazard ratio; PBO = Placebo HR = Hazard ratio; PBO = Placebo HR = Hazard ratio; PBO = Placebo Slide provided by Dr. Fred Saad Slide provided by Dr. Fred Saad
Slide provided by Dr. Fred Saad
Overall Survival for the AR-targeted Therapies in the Phase 3 Trials:
All three of the phase 3 clinical trials for the AR-targeted therapies have shown a statistically
significant overall survival benefit for men with nmCRPC. 5-7
Apalutamide Darolutamide Enzalutamide
(SPARTAN) 5 (ARAMIS) 6 (PROSPER) 7
Median OS Median OS Median OS
APA + ADT: 73.9 mos DARO + ADT: NR ENZA + ADT: 67.0 mos
PBO + ADT: 59.9 mos PBO + ADT: NR PBO + ADT: 56.3 mos
100
100
100
90
90
80 Darolutamide
80 80
70 60 Placebo 70
Overall Survival (%) 60 40 Placebo Apalutamide Overall Survival (%) 50 40 Overall Survival (%) 60 50 40 Placebo Enzalutamide
20 30 20 30 20
HR 0.78 (95% CI 0.64–0.96) 10 HR 0.69 (95% C 0.53–0.88) 10 HR 0.73 (95% CI 0.61–0.89)
p = 0.016 p = 0.003 p = 0.001
0 0 0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72
Months from randomisation Months since Randomization Months
No. at Risk No. at Risk No. at Risk
Apalutamide 806 791 774 758 739 717 691 658 625 593 558 499 376 269 181 100 47 19 4 0 Darolutamide 955 932 908 863 816 771 680 549 425 293 214 129 69 37 12 0 Enzalutamide 933 926 910 897 874 850 822 782 700 608 517 424 327 244 169 89 33 4 0
Placebo 401 392 385 373 358 339 328 306 286 263 240 204 156 114 82 38 21 6 2 0 Placebo 554 530 497 460 432 394 333 261 182 130 93 54 28 16 4 0 Placebo 468 467 459 444 428 404 381 363 321 274 219 177 140 106 64 30 16 3 0
ADT = Androgen deprivation therapy; APA = Apalutamide; DARO = Darolutamide; ENZA = Enzalutamide; HR = Hazard ratio; NR = Not reached; PBO = Placebo