Page 1 - Final Slides
P. 1
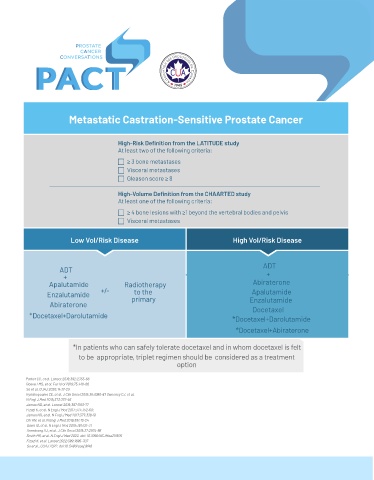
Metastatic Castration-Sensitive Prostate Cancer
High-Risk Definition from the LATITUDE study
At least two of the following criteria:
≥ 3 bone metastases
Visceral metastases
Gleason score ≥ 8
High-Volume Definition from the CHAARTED study
At least one of the following criteria:
≥ 4 bone lesions with ≥1 beyond the vertebral bodies and pelvis
Visceral metastases
Low Vol/Risk Disease High Vol/Risk Disease
ADT
ADT
+ +
Apalutamide Radiotherapy Abiraterone
to the
Enzalutamide +/- primary Apalutamide
Abiraterone Enzalutamide
Docetaxel
*Docetaxel+Darolutamide *Docetaxel+Darolutamide
*Docetaxel+Abiraterone
*In patients who can safely tolerate docetaxel and in whom docetaxel is felt
to be appropriate, triplet regimen should be considered as a treatment
option
Parker CC, et al. Lancet 2018;392:2353-66
Boeve LMS, et al. Eur Urol 2019;75:410-08
So et al. CUAJ 2020;14:17-23
Kyriakopoulos CE, et al. J Clin Oncol 2018;36:1080-87 Sweeney CJ, et al.
N Engl J Med 2015;373:737-46
James ND, et al. Lancet 2016;387:1163-77
Fizazi K, et al. N Engl J Med 2017;377:352-60;
James ND, et al. N Engl J Med 2017;377:338-51
Chi KN, et al. N Engl J Med 2019;381:13-24
Davis ID, et al. N Engl J Med 2019;381:121-31
Armstrong AJ, et al. J Clin Oncol 2019;37:2974-86
Smith MR, et al. N Engl J Med 2022. doi: 10.1056/NEJMoa2119115
Fizazi K, et al. Lancet 2022;399:1695-707
So et al., CUAJ 2022. doi:10.5489/cuaj.8148
4/4/22 6:18 PM
PACT-CUA FlowChart.indd 1 4/4/22 6:18 PM
PACT-CUA FlowChart.indd 1