Page 1 - mCSPC Card 05
P. 1
Management of Metastatic
Castration-Naïve and Castration-Sensitive
Prostate Cancer
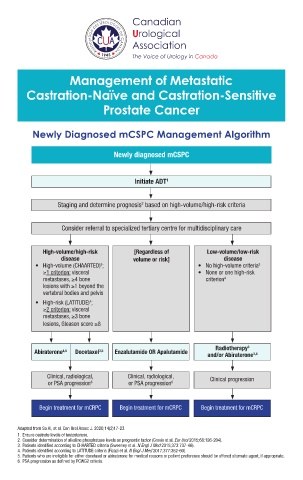
Newly Diagnosed mCSPC Management Algorithm
Newly diagnosed mCSPC
Initiate ADT 1
Staging and determine prognosis based on high-volume/high-risk criteria
2
Consider referral to specialized tertiary centre for multidisciplinary care
High-volume/high-risk [Regardless of Low-volume/low-risk
disease volume or risk] disease
• High-volume (CHAARTED) ; • No high-volume criteria 3
3
≥1 criterion: visceral • None or one high-risk
metastases, ≥4 bone criterion 4
lesions with ≥1 beyond the
vertebral bodies and pelvis
• High-risk (LATITUDE) ;
4
≥2 criterion: visceral
metastases, ≥3 bone
lesions, Gleason score ≥8
Radiotherapy 3
Abiraterone 4,5 Docetaxel 3,5 Enzalutamide OR Apalutamide
and/or Abiraterone 3,4
Clinical, radiological, Clinical, radiological, Clinical progression
or PSA progression 6 or PSA progression 6
Begin treatment for mCRPC Begin treatment for mCRPC Begin treatment for mCRPC
Adapted from So AI, et al. Can Urol Assoc J. 2020;14(2)17-23.
1. Ensure castrate levels of testosterone.
2. Consider determination of alkaline phosphatase levels as prognostic factor (Gravis et al. Eur Urol 2015;68:196-204).
3. Patients identified according to CHAARTED criteria (Sweeney et al. N Engl J Med 2015;373:737-46).
4. Patients identified according to LATITUDE criteria (Fizazi et al. N Engl J Med 2017;377:352-60).
5. Patients who are ineligible for either docetaxel or abiraterone for medical reasons or patient preference should be offered alternate agent, if appropriate.
6. PSA progression as defined by PCWG2 criteria.