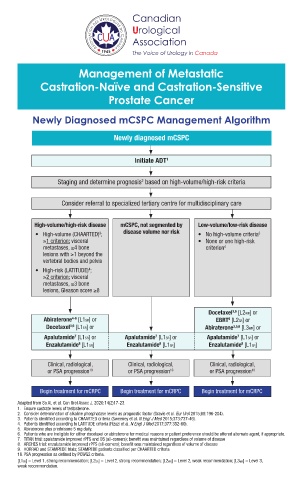
Page 1 - mCSPC Card 07
P. 1
Management of Metastatic
Castration-Naïve and Castration-Sensitive
Prostate Cancer
Newly Diagnosed mCSPC Management Algorithm
Newly diagnosed mCSPC
Initiate ADT 1
Staging and determine prognosis based on high-volume/high-risk criteria
2
Consider referral to specialized tertiary centre for multidisciplinary care
High-volume/high-risk disease mCSPC, not segmented by Low-volume/low-risk disease
3
• High-volume (CHAARTED) ; disease volume nor risk • No high-volume criteria 3
≥1 criterion: visceral • None or one high-risk
metastases, ≥4 bone criterion 4
lesions with ≥1 beyond the
vertebral bodies and pelvis
• High-risk (LATITUDE) ;
4
≥2 criterion: visceral
metastases, ≥3 bone
lesions, Gleason score ≥8
Docetaxel [L2WR] or
3,6
Abiraterone [L1SR] or EBRT [L2SR] or
4-6
9
Docetaxel [L1SR] or Abiraterone 3,5,6 [L3WR] or
3,6
Apalutamide [L1SR] or Apalutamide [L1SR] or Apalutamide [L1SR] or
7
7
7
8
Enzalutamide [L1SR] Enzalutamide [L1SR] Enzalutamide [L1SR]
8
8
Clinical, radiological, Clinical, radiological, Clinical, radiological,
or PSA progression 10 or PSA progression 10 or PSA progression 10
Begin treatment for mCRPC Begin treatment for mCRPC Begin treatment for mCRPC
Adapted from So AI, et al. Can Urol Assoc J. 2020;14(2)17-23.
1. Ensure castrate levels of testosterone.
2. Consider determination of alkaline phosphatase levels as prognostic factor (Gravis et al. Eur Urol 2015;68:196-204).
3. Patients identified according to CHAARTED criteria (Sweeney et al. N Engl J Med 2015;373:737-46).
4. Patients identified according to LATITUDE criteria (Fizazi et al. N Engl J Med 2017;377:352-60).
5. Abiraterone plus prednisone 5 mg daily.
6. Patients who are ineligible for either docetaxel or abiraterone for medical reasons or patient preference should be offered alternate agent, if appropriate.
7. TITAN trial: apalutamide improved rPFS and OS (all-comers); benefit was maintained regardless of volume of disease
8. ARCHES trial: enzalutamide improved rPFS (all-comers); benefit was maintained regardless of volume of disease
9. HORRAD and STAMPEDE trials; STAMPEDE patients classified per CHAARTED criteria
10. PSA progression as defined by PCWG2 criteria.
[L1SR] = Level 1, strong recommendation; [L2SR] = Level 2, strong recommendation; [L2WR] = Level 2, weak recommendation; [L3WR] = Level 3,
weak recommendation.