Page 2 - BC Management ToolCard
P. 2
Multidisciplinary Management
of Bladder Cancer
Treatment Tool
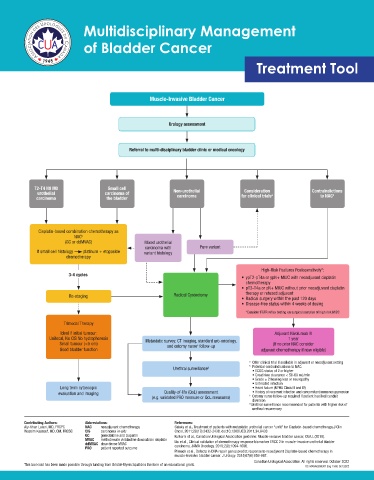
Muscle-Invasive Bladder Cancer
Urology assessment
Referral to multi-disciplinary bladder clinic or medical oncology
T2-T4 N0 M0 Small cell
urothelial carcinoma of Non-urothelial Consideration a Contraindictions
b
carcinoma the bladder carcinoma for clinical trials to NAC
Cisplatin-based combination chemotherapy as
NAC b
(GC or ddMVAC) Mixed urothelial
carcinoma with Pure variant
If small cell histology platinum + etoposide variant histology
chemotherapy
High-Risk Features Postoperatively*:
3-4 cycles
• ypT2-pT4a or ypN+ MIUC with neoadjuvant cisplatin
chemotherapy
• pT3-T4a or pN+ MIUC without prior neoadjuvant cisplatin
Re-staging Radical Cystectomy therapy or refused adjuvant
• Radical surgery within the past 120 days
• Disease-free status within 4 weeks of dosing
*Consider FGFR reflex testing on surgical samples of high risk MIBC
Trimodal Therapy
Ideal if initial tumour: Adjuvant Nivolumab IV
Unifocal, No CIS No hydrophoresis 1 year
Small tumour (<5 cm) Metastatic survey: CT imaging, standard uro-oncology, (If no prior NAC consider
and ostomy nurse follow-up
c
Good bladder function adjuvant chemotherapy if now eligible)
a Offer clinical trial if available in adjuvant or neoadjuvant setting
b Potential contraindications to NAC:
Urethral surveillance d • ECOG status of 2 or higher
• Creatinine clearance < 50-60 mL/min
• Grade ≥ 2 hearing loss or neuropathy
• Untreated infection
Long term cytoscopic • Heart failure (NYHA Class III and IV)
evaluation and imaging Quality-of-life (QoL) assessment • History of recurrent infection and concomitant immunosuppression
c
(e.g. validated PRO measure or QoL measures) Ostomy nurse follow-up required if patient has ileal conduit
diversion
d Urethral surveillance recommended for patients with higher risk of
urethral recurrencey
Contributing Authors: Abbreviations: References:
Aly-Khan Lalani, MD, FRCPC NAC neoadjuvant chemotherapy Galsky et al., Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy.J Clin
Wassim Kassouf, MD, CM, FRCSC CIS carcinoma in-situ Oncol. 2011;29(17):2432-2438. doi:10.1200/JCO.2011.34.8433
GC gemcitabine and cisplatin Kulkarni et al., Canadian Urological Association guideline: Muscle-invasive bladder cancer, CUAJ. (2019).
MVAC methotrexate vinblastine doxorubicin cisplatin
ddMVAC dose dense MVAC Liu et al., Clinical validation of chemotherapy response biomarker ERCC 2 in muscle-invasive urothelial bladder
PRO patient reported outcome carcinoma. JAMA Oncology. 2016;2(8):1094-1096.
Plimack et al., Defects in DNA repair genes predict response to neoadjuvant Cisplatin-based chemotherapy in
muscle-invasive bladder cancer. J Urology. 2015;67(6):959-967.
Canadian Urological Association. All rights reserved. October 2022
This tool card has been made possible through funding from Bristol-Myers Squibb in the form of an educational grant. BC-MANAGEMENT-Eng-1500-Oct.2022