Page 44 - TSC related Angiomyolipoma - Management Strategies and Case Studies
P. 44
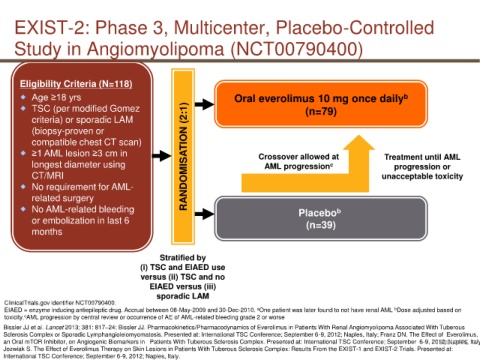
EXIST-2: Phase 3, Multicenter, Placebo-Controlled
Study in Angiomyolipoma (NCT00790400)
Eligibility Criteria (N=118)
Age ≥18 yrs Oral everolimus 10 mg once daily b
TSC (per modified Gomez (n=79)
criteria) or sporadic LAM
(biopsy-proven or
compatible chest CT scan)
≥1 AML lesion ≥3 cm in RANDOMISATION (2:1) Crossover allowed at Treatment until AML
longest diameter using AML progression c progression or
CT/MRI unacceptable toxicity
No requirement for AML-
related surgery
No AML-related bleeding Placebo
b
or embolization in last 6 (n=39)
months
Stratified by
(i) TSC and EIAED use
versus (ii) TSC and no
EIAED versus (iii)
sporadic LAM
ClinicalTrials.gov identifier NCT00790400.
b
a
EIAED = enzyme inducing antiepileptic drug. Accrual between 08-May-2009 and 30-Dec-2010. One patient was later found to not have renal AML Dose adjusted based on
toxicity. AML progression by central review or occurrence of AE of AML-related bleeding grade 2 or worse
c
Bissler JJ et al. Lancet 2013; 381: 817–24; Bissler JJ. Pharmacokinetics/Pharmacodynamics of Everolimus in Patients With Renal Angiomyolipoma Associated With Tuberous
Sclerosis Complex or Sporadic Lymphangioleiomyomatosis. Presented at: International TSC Conference; September 6-9, 2012; Naples, Italy; Franz DN. The Effect of Everolimus,
an Oral mTOR Inhibitor, on Angiogenic Biomarkers in Patients With Tuberous Sclerosis Complex. Presented at: International TSC Conference; September 6-9, 2012; Naples, Italy;
13SOL017E
Jozwiak S. The Effect of Everolimus Therapy on Skin Lesions in Patients With Tuberous Sclerosis Complex: Results From the EXIST-1 and EXIST-2 Trials. Presented at:
International TSC Conference; September 6-9, 2012; Naples, Italy.