Page 46 - Monitoring Prostate Cancer to Guide Treatment Decision-Making
P. 46
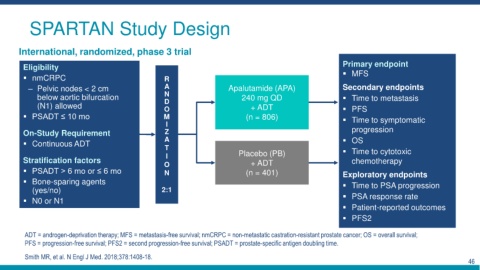
SPARTAN Study Design
International, randomized, phase 3 trial
Eligibility Primary endpoint
nmCRPC R MFS
– Pelvic nodes < 2 cm A Apalutamide (APA) Secondary endpoints
below aortic bifurcation N 240 mg QD Time to metastasis
D
(N1) allowed O + ADT PFS
PSADT ≤ 10 mo M (n = 806) Time to symptomatic
I
On-Study Requirement Z progression
Continuous ADT A OS
T Time to cytotoxic
I Placebo (PB)
Stratification factors O + ADT chemotherapy
PSADT > 6 mo or ≤ 6 mo N (n = 401) Exploratory endpoints
Bone-sparing agents Time to PSA progression
(yes/no) 2:1
N0 or N1 PSA response rate
Patient-reported outcomes
PFS2
ADT = androgen-deprivation therapy; MFS = metastasis-free survival; nmCRPC = non-metastatic castration-resistant prostate cancer; OS = overall survival;
PFS = progression-free survival; PFS2 = second progression-free survival; PSADT = prostate-specific antigen doubling time.
Smith MR, et al. N Engl J Med. 2018;378:1408-18.
46