Page 37 - Update on the treatment options in Kidney Cancer
P. 37
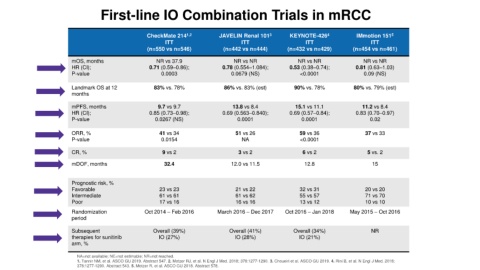
First-line IO Combination Trials in mRCC
CheckMate 214 1,2 JAVELIN Renal 101 3 KEYNOTE-426 4 IMmotion 151 5
ITT ITT ITT ITT
(n=550 vs n=546) (n=442 vs n=444) (n=432 vs n=429) (n=454 vs n=461)
mOS, months NR vs 37.9 NR vs NR NR vs NR NR vs NR
HR (CI); 0.71 (0.59–0.86); 0.78 (0.554–1.084); 0.53 (0.38–0.74); 0.81 (0.63–1.03)
P-value 0.0003 0.0679 (NS) <0.0001 0.09 (NS)
Landmark OS at 12 83% vs. 78% 86% vs. 83% (est) 90% vs. 78% 80% vs. 79% (est)
months
mPFS, months 9.7 vs 9.7 13.8 vs 8.4 15.1 vs 11.1 11.2 vs 8.4
HR (CI); 0.85 (0.73–0.98); 0.69 (0.563–0.840); 0.69 (0.57–0.84); 0.83 (0.70–0.97)
P-value 0.0267 (NS) 0.0001 0.0001 0.02
ORR, % 41 vs 34 51 vs 26 59 vs 36 37 vs 33
P-value 0.0154 NA <0.0001
CR, % 9 vs 2 3 vs 2 6 vs 2 5 vs. 2
mDOF, months 32.4 12.0 vs 11.5 12.8 15
Prognostic risk, %
Favorable 23 vs 23 21 vs 22 32 vs 31 20 vs 20
Intermediate 61 vs 61 61 vs 62 55 vs 57 71 vs 70
Poor 17 vs 16 16 vs 16 13 vs 12 10 vs 10
Randomization Oct 2014 – Feb 2016 March 2016 – Dec 2017 Oct 2016 – Jan 2018 May 2015 – Oct 2016
period
Subsequent Overall (39%) Overall (41%) Overall (34%) NR
therapies for sunitinib IO (27%) IO (28%) IO (21%)
arm, %
NA=not available; NE=not estimable; NR=not reached.
1. Tannir NM, et al. ASCO GU 2019. Abstract 547. 2. Motzer RJ, et al. N Engl J Med. 2018; 378:1277-1290. 3. Choueiri et al. ASCO GU 2019. 4. Rini B, et al. N Engl J Med. 2018;
378:1277-1290. Abstract 543. 5. Motzer R, et al. ASCO GU 2018. Abstract 578.
37