Page 57 - Practical Approaches to Managing Castration-Resistant Prostate Cancer (CRPC)
P. 57
Rash
Description of Apalutamide-Associated Rash (SPARTAN trial)
• Most commonly described as macular or maculo-papular
• Occurred in ~25% of patients treated with apalutamide (rate of grade 3 rash
was ~5%)
• Onset was ~80 days
• Resolved ~60 days from onset for 80% of patients; grade 1 rashes resolved
more quickly (36 days)
• Dose modifications due to rash in SPARTAN
– 28% dose interruption
– 12% dose reduction
– 9% discontinuation
• Rash recurred with re-challenge in ~50%, with no serious allergic reactions
Assessment and Treatment of Maculo-Papular Rash Associated with Apalutamide
• Rash characterized by the presence of macules (flat) and papules (elevated)
• Frequently affecting the upper trunk, spreading centripetally and associated
with pruritus
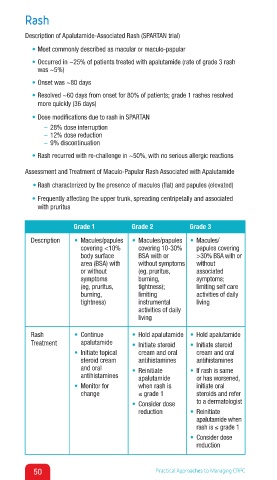
Grade 1 Grade 2 Grade 3
Description • Macules/papules • Macules/papules • Macules/
covering <10% covering 10-30% papules covering
body surface BSA with or >30% BSA with or
area (BSA) with without symptoms without
or without (eg, pruritus, associated
symptoms burning, symptoms;
(eg, pruritus, tightness); limiting self care
burning, limiting activities of daily
tightness) instrumental living
activities of daily
living
Rash • Continue • Hold apalutamide • Hold apalutamide
Treatment apalutamide • Initiate steroid • Initiate steroid
• Initiate topical cream and oral cream and oral
steroid cream antihistamines antihistamines
and oral • Reinitiate • If rash is same
antihistamines apalutamide or has worsened,
• Monitor for when rash is initiate oral
change ≤ grade 1 steroids and refer
• Consider dose to a dermatologist
reduction • Reinitiate
apalutamide when
rash is ≤ grade 1
• Consider dose
reduction
50 Practical Approaches to Managing CRPC