Page 31 - Managing Advanced Urothelial Carcinoma in 2020: Canadian Consensus Updates
P. 31
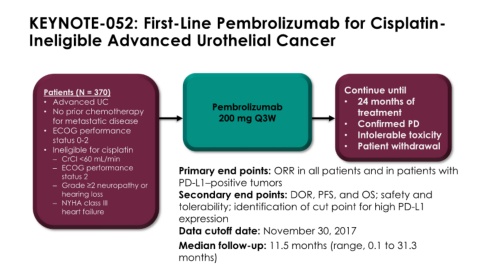
KEYNOTE-052: First-Line Pembrolizumab for Cisplatin-
Ineligible Advanced Urothelial Cancer
Patients (N = 370) Continue until
• Advanced UC Pembrolizumab • 24 months of
• No prior chemotherapy treatment
for metastatic disease 200 mg Q3W • Confirmed PD
• ECOG performance • Intolerable toxicity
status 0-2
• Ineligible for cisplatin • Patient withdrawal
– CrCl <60 mL/min
– ECOG performance Primary end points: ORR in all patients and in patients with
status 2
– Grade ≥2 neuropathy or PD-L1–positive tumors
hearing loss Secondary end points: DOR, PFS, and OS; safety and
– NYHA class III tolerability; identification of cut point for high PD-L1
heart failure
expression
Data cutoff date: November 30, 2017
Median follow-up: 11.5 months (range, 0.1 to 31.3
months)