Page 49 - CUA 2020_Onco_Prostate
P. 49
Moderated Posters 4: Prostate Cancer I
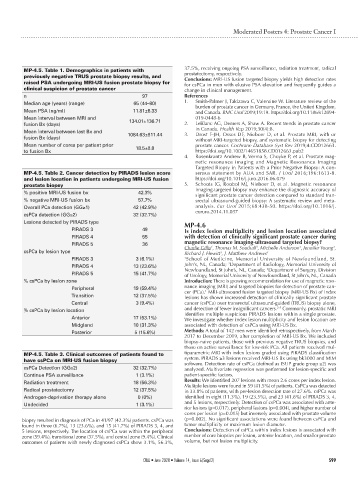
MP-4.5. Table 1. Demographics in patients with 37.5%, receiving ongoing PSA surveillance, radiation treatment, radical
prostatectomy, respectively.
previously negative TRUS prostate biopsy results, and Conclusions: MRI-US fusion targeted biopsy yields high detection rates
raised PSA undergoing MRI-US fusion prostate biopsy for for csPCa in men with elusive PSA elevation and frequently guides a
clinical suspicion of prostate cancer change in clinical management.
n 97 References
Median age (years) (range) 65 (44–80) 1. Smith-Palmer J, Takizawa C, Valentine W. Literature review of the
burden of prostate cancer in Germany, France, the United Kingdom,
Mean PSA (ng/ml) 11.81±6.33 and Canada. BMC Urol 2019;19:19. https://doi.org/10.1186/s12894-
Mean interval between MRI and 134.01±136.71 019-0448-6
fusion Bx (days) 2. LeBlanc AG, Demers A, Shaw A. Recent trends in prostate cancer
Mean interval between last Bx and 1084.63±811.44 3. in Canada. Health Rep 2019;30(4):8.
Drost F-JH, Osses DF, Nieboer D, et al. Prostate MRI, with or
fusion Bx (days) without MRI-targeted biopsy, and systematic biopsy for detecting
Mean number of cores per patient prior 18.5±8.8 prostate cancer. Cochrane Database Syst Rev 2019;4:CD012663.
to fusion Bx https://doi.org/10.1002/14651858.CD012663.pub2
4. Rosenkrantz Andrew B, Verma S, Choyke P, et al. Prostate mag-
netic resonance imaging and Magnetic Resonance Imaging
Targeted Biopsy in Patients with a Prior Negative Biopsy: A con-
MP-4.5. Table 2. Cancer detection by PIRADS lesion score sensus statement by AUA and SAR. J Urol 2016;196:1613-8.
and lesion location in patients undergoing MRI-US fusion https://doi.org/10.1016/j.juro.2016.06.079
prostate biopsy 5. Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance
% positive MRI-US fusion bx 42.3% imaging-targeted biopsy may enhance the diagnostic accuracy of
significant prostate cancer detection compared to standard tran-
% negative MRI-US fusion bx 57.7% srectal ultrasound-guided biopsy: A systematic review and meta-
Overall PCa detection (GG≥1) 42 (42.9%) analysis. Eur Urol 2015;68:438-50. https://doi.org/10.1016/j.
csPCa detection (GG≥2) 32 (32.7%) eururo.2014.11.037
Lesions detected by PIRADS type MP-4.6
PIRADS 3 49 Is index lesion multiplicity and lesion location associated
PIRADS 4 55 with detection of clinically significant prostate cancer during
PIRADS 5 36 magnetic resonance imaging-ultrasound targeted biopsy? 2
1
2
1
csPCa by lesion type Charlie Gillis , Thomas M. Southall , Michelle Anderson , Jennifer Young ,
Richard J. Hewitt , J. Matthew Andrews
3
3
PIRADS 3 3 (6.1%) 1 School of Medicine, Memorial University of Newfoundland, St.
2
PIRADS 4 13 (23.6%) John’s, NL, Canada; Department of Radiology, Memorial University of
Newfoundland, St John’s, NL, Canada; Department of Surgery, Division
3
PIRADS 5 15 (41.7%) of Urology, Memorial University of Newfoundland, St John’s, NL, Canada
% csPCa by lesion zone Introduction: There is growing recommendation for use of magnetic reso-
Peripheral 19 (59.4%) nance imaging (MRI) and targeted biopsies for detection of prostate can-
cer (PCa). MRI-ultrasound fusion targeted biopsy (MRI-US Bx) of index
1
Transition 12 (37.5%) lesions has shown increased detection of clinically significant prostate
Central 3 (9.4%) cancer (csPCa) over transrectal ultrasound-guided (TRUS) biopsy alone,
2-5
% csPCa by lesion location and detection of fewer insignificant cancers. Commonly, prostatic MRI
identifies multiple suspicious PIRADS lesions within a single prostate.
Anterior 17 (53.1%) We investigate whether index lesion multiplicity and lesion location are
Midgland 10 (31.3%) associated with detection of csPCa using MRI-US Bx.
Posterior 5 (15.6%) Methods: A total of 142 men were identified retrospectively, from March
2017 to December 2019, after completion of MRI-US Bx. We included
biopsy-naive patients, those with previous negative TRUS biopsies, and
those on active surveillance for low-risk PCa. All patients received mul-
MP-4.5. Table 3. Clinical outcomes of patients found to tiparametric MRI with index lesions graded using PIRADS classification
have csPCa on MRI-US fusion biopsy system. PIRADS ≥3 lesions received MRI-US Bx using bk3000 and MIM
software. Detection rate of csPCa (defined as ISUP grade group ≥2) was
csPCa Detection (GG≥2) 32 (32.7%) analyzed. Multivariate regression was performed for lesion-specific and
Continue PSA surveillance 1 (3.1%) patient-specific factors.
Radiation treatment 18 (56.3%) Results: We identified 207 lesions with mean 2.6 cores per index lesion.
Multiple lesions were found in 59 (41.5%) of patients. CsPCa was detected
Radical prostatectomy 12 (37.5%) in 33.8% of patients, with per-lesion detection rate of 27.6%. csPCa was
Androgen-deprivation therapy alone 0 (0%) identified in eight (11.3%), 19 (23.5%), and 23 (41.8%) of PIRADS 3, 4,
Undecided 1 (3.1%) and 5 lesions, respectively. Detection of csPCa was associated with ante-
rior lesions (p=0.017), peripheral lesions (p=0.004), and higher number of
cores per lesion (p=0.015) but inversely associated with prostate volume
biopsy resulted in diagnosis of PCa in 41/97 (42.3%) patients. csPCa was (p=0.002). No significant associations were found between csPCa and
found in three (6.7%), 13 (23.6%), and 15 (41.7%) of PIRADS 3, 4, and tumor multiplicity or maximum lesion diameter.
5 lesions, respectively. The location of csPCa was within the peripheral Conclusions: Detection of csPCa within index lesions is associated with
zone (59.4%), transitional zone (37.5%), and central zone (9.4%). Clinical number of core biopsies per lesion, anterior location, and smaller prostate
outcomes of patients with newly diagnosed csPCa show 3.1%, 56.3%, volume, but not lesion multiplicity.
CUAJ • June 2020 • Volume 14, Issue 6(Suppl2) S99