Page 92 - Flipbook
P. 92
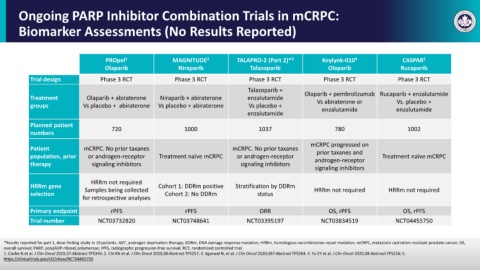
Ongoing PARP Inhibitor Combination Trials in mCRPC:
Biomarker Assessments (No Results Reported)
PROpel 1 MAGNITUDE 2 TALAPRO-2 (Part 2)* 3 Keylynk-010 4 CASPAR 5
Olaparib Niraparib Talazoparib Olaparib Rucaparib
Trial design Phase 3 RCT Phase 3 RCT Phase 3 RCT Phase 3 RCT Phase 3 RCT
Talazoparib +
Treatment Olaparib + abiraterone Niraparib + abiraterone enzalutamide Olaparib + pembrolizumab Rucaparib + enzalutamide
Vs. placebo +
Vs abiraterone or
groups Vs placebo + abiraterone Vs placebo + abiraterone Vs placebo + enzalutamide enzalutamide
enzalutamide
Planned patient 720 1000 1037 780 1002
numbers
mCRPC progressed on
Patient mCRPC. No prior taxanes mCRPC. No prior taxanes prior taxanes and
population, prior or androgen-receptor Treatment naïve mCRPC or androgen-receptor Treatment naïve mCRPC
therapy signaling inhibitors signaling inhibitors androgen-receptor
signaling inhibitors
HRRm not required
HRRm gene Samples being collected Cohort 1: DDRm positive Stratification by DDRm HRRm not required HRRm not required
selection Cohort 2: No DDRm status
for retrospective analyses
Primary endpoint rPFS rPFS ORR OS, rPFS OS, rPFS
Trial number NCT03732820 NCT03748641 NCT03395197 NCT03834519 NCT04455750
*Results reported for part 1, dose-finding study in 19 patients. ADT, androgen deprivation therapy; DDRm, DNA damage response mutation; HRRm, homologous recombination repair mutation; mCRPC, metastatic castration-resistant prostate cancer; OS,
overall survival; PARP, poly(ADP-ribose) polymerase; rPFS, radiographic progression-free survival; RCT, randomized controlled trial.
1. Clarke N et al. J Clin Oncol 2019;37:Abstract TPS340; 2. Chi KN et al. J Clin Oncol 2020;38:Abstract TPS257; 3. Agarwal N, et al. J Clin Oncol 2020;387:Abstract TPS264. 4. Yu EY et al. J Clin Oncol 2020;38:Abstract TPS256; 5.
https://clinicaltrials.gov/ct2/show/NCT04455750