Page 61 - CUA Adv Prostate Ca Drug Acccess Listing
P. 61
QUEBEC Link to Patient Assistance Programs
Funding:
Medications that are taken at home may be covered by the provincial drug benefit plan or by private insurance plans.
Formulary:
RAMQ Formulary https://www.ramq.gouv.qc.ca/fr/a-propos/liste-medicaments
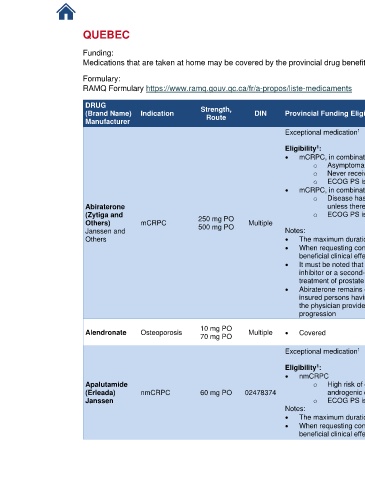
DRUG Strength, References
(Brand Name) Indication Route DIN Provincial Funding Eligibility Criteria
Manufacturer
Exceptional medication 1 1. RAMQ List of
Medications
1
Eligibility : [9-21]
• mCRPC, in combination with prednisone
o Asymptomatic or mildly symptomatic after an anti-androgen treatment has failed;
o Never received docetaxel-based chemotherapy;
o ECOG PS is 0 or 1.
• mCRPC, in combination with prednisone
o Disease has progressed during or following docetaxel-based chemotherapy,
Abiraterone unless there is a contraindication or a serious intolerance;
(Zytiga and 250 mg PO o ECOG PS is ≤ 2.
Others) mCRPC 500 mg PO Multiple
Janssen and Notes:
Others • The maximum duration of each authorization is four months
• When requesting continuation of treatment, the physician must provide evidence of a
beneficial clinical effect by the absence of disease progression
• It must be noted that abiraterone is not authorized after failure with an androgen synthesis
inhibitor or a second-generation androgen receptor inhibitor if it was administered for
treatment of prostate cancer
• Abiraterone remains covered by the basic prescription drug insurance plan for those
insured persons having used this drug in the three months before 10 July 2019, insofar as
the physician provides evidence of a beneficial clinical effect by the absence of disease
progression
1. RAMQ List of
Alendronate Osteoporosis 10 mg PO Multiple • Covered Medications
70 mg PO
[9-21]
Exceptional medication 1 1. RAMQ List of
Medications
Eligibility : [9-21]
1
• nmCRPC
Apalutamide o High risk of developing distant metastases (PSADT ≤ 10 months) despite an
(Erleada) nmCRPC 60 mg PO 02478374 androgenic deprivation treatment
Janssen o ECOG PS is 0 or 1
Notes:
• The maximum duration of each authorization is four months
• When requesting continuation of treatment, the physician must provide evidence of a
beneficial clinical effect defined by the absence of disease progression
Page 31 | © Canadian Urological Association
v.01-SEP-2021