Page 54 - Demo
P. 54
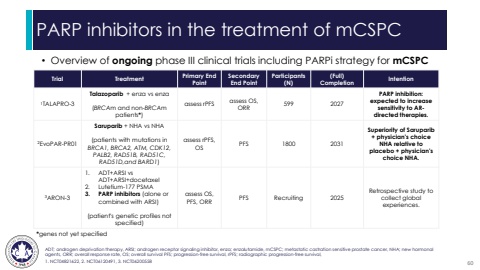
PARP inhibitors in the treatment of mCSPC%u2022 Overview of ongoing phase III clinical trials including PARPi strategy for mCSPCADT; androgen deprivation therapy, ARSI; androgen receptor signaling inhibitor, enza; enzalutamide, mCSPC; metastatic castration sensitive prostate cancer, NHA; new hormonal agents, ORR; overall response rate, OS; overall survival PFS; progression-free survival, rPFS; radiographic progression-free survival, 1. NCT04821622, 2. NCT06120491, 3. NCT06200558 Trial Treatment Primary End PointSecondary End PointParticipants (N)(Full) Completion Intention1TALAPRO-3 Talazoparib + enza vs enza(BRCAm and non-BRCAmpatients*)assess rPFS assess OS, ORR 599 2027PARP inhibition: expected to increase sensitivity to ARdirected therapies. 2EvoPAR-PR01Saruparib + NHA vs NHA(patients with mutations in BRCA1, BRCA2, ATM, CDK12, PALB2, RAD51B, RAD51C, RAD51D,and BARD1)assess rPFS, OS PFS 1800 2031Superiority of Saruparib+ physician's choice NHA relative to placebo + physician's choice NHA.3ARON-31. ADT+ARSI vs ADT+ARSI+docetaxel2. Lutetium-177 PSMA3. PARP inhibitors (alone or combined with ARSI)(patient's genetic profiles not specified)assess OS, PFS, ORR PFS Recruiting 2025Retrospective study to collect global experiences.*genes not yet specified60