Page 25 - Demo
P. 25
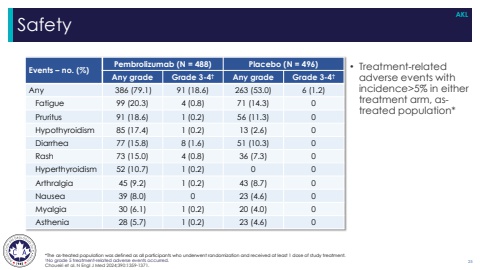
Safety*The as-treated population was defined as all participants who underwent randomization and received at least 1 dose of study treatment.%u2020No grade 5 treatment-related adverse events occurred.Choueiri et al. N Engl J Med 2024;390:1359-1371.Events %u2013 no. (%) Pembrolizumab (N = 488) Placebo (N = 496)Any grade Grade 3-4%u2020 Any grade Grade 3-4%u2020Any 386 (79.1) 91 (18.6) 263 (53.0) 6 (1.2)Fatigue 99 (20.3) 4 (0.8) 71 (14.3) 0Pruritus 91 (18.6) 1 (0.2) 56 (11.3) 0Hypothyroidism 85 (17.4) 1 (0.2) 13 (2.6) 0Diarrhea 77 (15.8) 8 (1.6) 51 (10.3) 0Rash 73 (15.0) 4 (0.8) 36 (7.3) 0Hyperthyroidism 52 (10.7) 1 (0.2) 0 0Arthralgia 45 (9.2) 1 (0.2) 43 (8.7) 0Nausea 39 (8.0) 0 23 (4.6) 0Myalgia 30 (6.1) 1 (0.2) 20 (4.0) 0Asthenia 28 (5.7) 1 (0.2) 23 (4.6) 025AKL%u2022 Treatment-related adverse events with incidence>5% in either treatment arm, astreated population*