Page 16 - Flipbook
P. 16
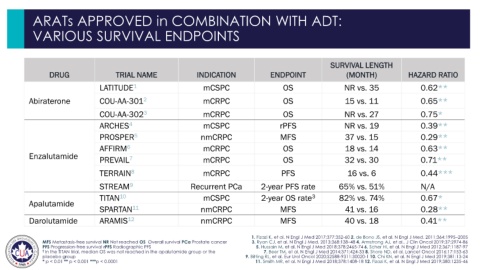
ARATs APPROVED in COMBINATION WITH ADT:
VARIOUS SURVIVAL ENDPOINTS
SURVIVAL LENGTH
DRUG TRIAL NAME INDICATION ENDPOINT (MONTH) HAZARD RATIO
LATITUDE 1 mCSPC OS NR vs. 35 0.62**
Abiraterone COU-AA-301 2 mCRPC OS 15 vs. 11 0.65**
COU-AA-302 3 mCRPC OS NR vs. 27 0.75*
ARCHES 4 mCSPC rPFS NR vs. 19 0.39**
PROSPER 5 nmCRPC MFS 37 vs. 15 0.29**
AFFIRM 6 mCRPC OS 18 vs. 14 0.63**
Enzalutamide PREVAIL 7 mCRPC OS 32 vs. 30 0.71**
TERRAIN 8 mCRPC PFS 16 vs. 6 0.44***
STREAM 9 Recurrent PCa 2-year PFS rate 65% vs. 51% N/A
TITAN 10 mCSPC 2-year OS rate 3 82% vs. 74% 0.67*
Apalutamide
SPARTAN 11 nmCRPC MFS 41 vs. 16 0.28**
Darolutamide ARAMIS 12 nmCRPC MFS 40 vs. 18 0.41**
1. Fizazi K, et al. N Engl J Med 2017;377:352-60 2. de Bono JS, et al. N Engl J Med. 2011;364:1995–2005
MFS Metastasis-free survival NR Not reached OS Overall survival PCa Prostate cancer 3. Ryan CJ, et al. N Engl J Med. 2013;368:138–48 4. Armstrong AJ, et al.. J Clin Oncol 2019;37:2974-86
PFS Progression-free survival rPFS Radiographic PFS 5. Hussain M, et al. N Engl J Med 2018;378:2465-74 6. Scher HI, et al. N Engl J Med 2012;367:1187-97
† In the TITAN trial, median OS was not reached in the apalutamide group or the 7. Beer TM, et al. N Engl J Med 2014;371:424-33 8. Shore ND, et al. Lancet Oncol 2016;17:153-63
placebo group 9. Bitting RL, et al. Eur Urol Oncol 2020;S2588-9311:30020-1 10. Chi KN, et al. N Engl J Med 2019;381:13-24
* p < 0.01 ** p < 0.001 ***p < 0.0001 11. Smith MR, et al. N Engl J Med 2018;378:1408-18 12. Fizazi K, et al. N Engl J Med 2019;380:1235-46