Page 6 - Flipbook
P. 6
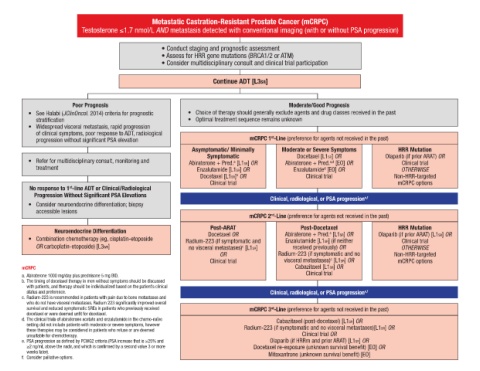
Metastatic Castration-Resistant Prostate Cancer (mCRPC)
Testosterone ≤1.7 nmol/L AND metastasis detected with conventional imaging (with or without PSA progression)
• Conduct staging and prognostic assessment
• Assess for HRR gene mutations (BRCA1/2 or ATM)
• Consider multidisciplinary consult and clinical trial participation
Continue ADT [L3sr]
Poor Prognosis Moderate/Good Prognosis
• See Halabi (JClinOncol. 2014) criteria for prognostic • Choice of therapy should generally exclude agents and drug classes received in the past
stratification • Optimal treatment sequence remains unknown
• Widespread visceral metastasis, rapid progression
of clinical symptoms, poor response to ADT, radiological
st
progression without significant PSA elevation mCRPC 1 -Line (preference for agents not received in the past)
Asymptomatic/ Minimally Moderate or Severe Symptoms HRR Mutation
Symptomatic Docetaxel [L1SR] OR Olaparib (if prior ARAT) OR
• Refer for multidisciplinary consult, monitoring and Abiraterone + Pred. [L1SR] OR Abiraterone + Pred. [EO] OR Clinical trial
a
a,d
treatment Enzalutamide [L1SR] OR Enzalutamide [EO] OR OTHERWISE
d
Docetaxel [L1SR] OR Clinical trial Non-HRR-targeted
b
Clinical trial mCRPC options
No response to 1 -line ADT or Clinical/Radiological
st
Progression Without Significant PSA Elevations
Clinical, radiological, or PSA progression e,f
• Consider neuroendocrine differentiation; biopsy
accessible lesions
mCRPC 2 -Line (preference for agents not received in the past)
nd
Post-ARAT Post-Docetaxel HRR Mutation
Neuroendocrine Differentiation Docetaxel OR Abiraterone + Pred. [L1SR] OR Olaparib (if prior ARAT) [L1SR] OR
a
• Combination chemotherapy (eg, cisplatin-etoposide Radium-223 (if symptomatic and Enzalutamide [L1SR] (if neither Clinical trial
OR carboplatin-etoposide) [L3WR] no visceral metastases) [L1SR] received previously) OR OTHERWISE
c
OR Radium-223 (if symptomatic and no Non-HRR-targeted
c
Clinical trial visceral metastases) [L1SR] OR mCRPC options
mCRPC Cabazitaxel [L1SR] OR
a. Abiraterone 1000 mg/day plus prednisone 5 mg BID. Clinical trial
b. The timing of docetaxel therapy in men without symptoms should be discussed
with patients, and therapy should be individualized based on the patient’s clinical
status and preference. Clinical, radiological, or PSA progression e,f
c. Radium-223 is recommended in patients with pain due to bone metastases and
who do not have visceral metastases. Radium 223 significantly improved overall
survival and reduced symptomatic SREs in patients who previously received mCRPC 3 -Line (preference for agents not received in the past)
rd
docetaxel or were deemed unfit for docetaxel.
d. The clinical trials of abiraterone acetate and enzalutamide in the chemo-naïve Cabazitaxel (post-docetaxel) [L1SR] OR
setting did not include patients with moderate or severe symptoms, however Radium-223 (if symptomatic and no visceral metastases)[L1SR] OR
these therapies may be considered in patients who refuse or are deemed
unsuitable for chemotherapy. Clinical trial OR
e. PSA progression as defined by PCWG2 criteria (PSA increase that is ≥25% and Olaparib (if HRRm and prior ARAT) [L1SR] OR
≥2 ng/mL above the nadir, and which is confirmed by a second value 3 or more Docetaxel re-exposure (unknown survival benefit) [EO] OR
weeks later). Mitoxantrone (unknown survival benefit) [EO]
f. Consider palliative options.