Page 70 - Demo
P. 70
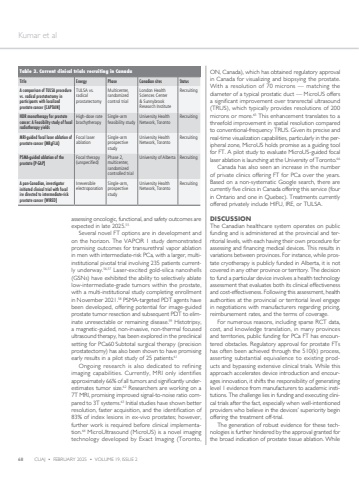
68 CUAJ %u2022 FEBRUARY 2025 %u2022 VOLUME 19, ISSUE 2 Kumar et alassessing oncologic, functional, and safety outcomes are expected in late 2025.55Several novel FT options are in development and on the horizon. The VAPOR 1 study demonstrated promising outcomes for transurethral vapor ablation in men with intermediate-risk PCa, with a larger, multiinstitutional pivotal trial involving 235 patients currently underway.56,57 Laser-excited gold-silica nanoshells (GSNs) have exhibited the ability to selectively ablate low-intermediate-grade tumors within the prostate, with a multi-institutional study completing enrollment in November 2021.58 PSMA-targeted PDT agents have been developed, offering potential for image-guided prostate tumor resection and subsequent PDT to eliminate unresectable or remaining disease.59 Histotripsy, a magnetic-guided, non-invasive, non-thermal focused ultrasound therapy, has been explored in the preclinical setting for PCa60 Subtotal surgical therapy (precision prostatectomy) has also been shown to have promising early results in a pilot study of 25 patients.61Ongoing research is also dedicated to refining imaging capabilities. Currently, MRI only identifies approximately 66% of all tumors and significantly underestimates tumor size.62 Researchers are working on a 7T MRI, promising improved signal-to-noise ratio compared to 3T systems.63 Initial studies have shown better resolution, faster acquisition, and the identification of 83% of index lesions in ex-vivo prostates; however, further work is required before clinical implementation.64 MicroUltrasound (MicroUS) is a novel imaging technology developed by Exact Imaging (Toronto, ON, Canada), which has obtained regulatory approval in Canada for visualizing and biopsying the prostate. With a resolution of 70 microns %u2014 matching the diameter of a typical prostatic duct %u2014 MicroUS offers a significant improvement over transrectal ultrasound (TRUS), which typically provides resolutions of 200 microns or more.65 This enhancement translates to a threefold improvement in spatial resolution compared to conventional-frequency TRUS. Given its precise and real-time visualization capabilities, particularly in the peripheral zone, MicroUS holds promise as a guiding tool for FT. A pilot study to evaluate MicroUS-guided focal laser ablation is launching at the University of Toronto.66Canada has also seen an increase in the number of private clinics offering FT for PCa over the years. Based on a non-systematic Google search, there are currently five clinics in Canada offering this service (four in Ontario and one in Quebec). Treatments currently offered privately include HIFU, IRE, or TULSA.DISCUSSIONThe Canadian healthcare system operates on public funding and is administered at the provincial and territorial levels, with each having their own procedure for assessing and financing medical devices. This results in variations between provinces. For instance, while prostate cryotherapy is publicly funded in Alberta, it is not covered in any other province or territory. The decision to fund a particular device involves a health technology assessment that evaluates both its clinical effectiveness and cost-effectiveness. Following this assessment, health authorities at the provincial or territorial level engage in negotiations with manufacturers regarding pricing, reimbursement rates, and the terms of coverage.For numerous reasons, including sparse RCT data, cost, and knowledge translation, in many provinces and territories, public funding for PCa FT has encountered obstacles. Regulatory approval for prostate FTs has often been achieved through the 510(k) process, asserting substantial equivalence to existing products and bypassing extensive clinical trials. While this approach accelerates device introduction and encourages innovation, it shifts the responsibility of generating level 1 evidence from manufacturers to academic institutions. The challenge lies in funding and executing clinical trials after the fact, especially when well-intentioned providers who believe in the devices%u2019 superiority begin offering the treatment off-trial.The generation of robust evidence for these technologies is further hindered by the approval granted for the broad indication of prostate tissue ablation. While Table 3. Current clinical trials recruiting in CanadaTitle Energy Phase Canadian sites StatusA comparison of TULSA procedure vs. radical prostatectomy in participants with localized prostate cancer (CAPTAIN)TULSA vs. radical prostatectomyMulticenter, randomized control trialLondon Health Sciences Center & Sunnybrook Research InstituteRecruitingHDR monotherapy for prostate cancer: A Feasibility study of focal radiotherapy yields High-dose rate brachytherapySingle-arm feasibility studyUniversity Health Network, TorontoRecruitingMRI-guided focal laser ablation of prostate cancer (MRgFLA)Focal laser ablationSingle-arm prospective studyUniversity Health Network, TorontoRecruitingPSMA-guided ablation of the prostate (P-GAP)Focal therapy (unspecified)Phase 2, multicenter, randomized controlled trialUniversity of Alberta RecruitingA pan-Canadian, investigator initiated clinical trial with focal ire directed to intermediate-risk prostate cancer (WIRED)Irreversible electroporationSingle-arm, prospective studyUniversity Health Network, TorontoRecruiting