Page 5 - Flipbook
P. 5
Consensus: Managing prostate cancer during COVID-19
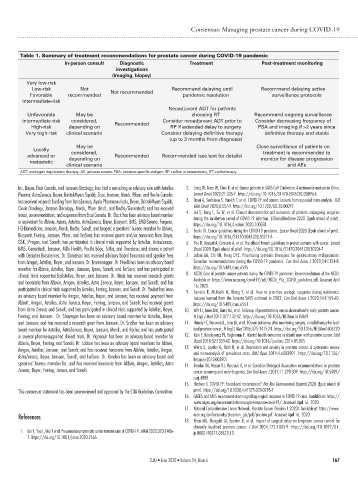
Table 1. Summary of treatment recommendations for prostate cancer during COVID-19 pandemic
In-person consult Diagnostic Treatment Post-treatment monitoring
investigations
(imaging, biopsy)
Very low-risk
Low-risk Not Recommend delaying until Recommend delaying active
Favorable recommended Not recommended pandemic resolution surveillance protocols
intermediate-risk
Neoadjuvant ADT for patients
Unfavorable May be choosing RT Recommend ongoing surveillance
intermediate-risk considered, Recommended Consider neoadjuvant ADT prior to Consider decreasing frequency of
High-risk depending on RP if extended delay to surgery PSA and imaging if >2 years since
Very high-risk clinical scenario Consider delaying definitive therapy definitive therapy and stable
(up to 3 months from diagnosis)
May be Close surveillance of patients on
Locally
treatment is recommended to
considered,
advanced or depending on Recommended Recommended (see text for details) monitor for disease progression
metastatic
clinical scenario and AEs
ADT: androgen deprivation therapy; AE: adverse events; PSA: prostate-specific antigen; RP: radical prostatectomy; RT: radiotherapy.
Inc, Bayer, Eisai Canada, and Janssen Oncology; has had a consulting or advisory role with Astellas 2. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China.
Pharma; AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Janssen, Merck, Pfizer, and Roche Canada; Lancet Oncol 2020;21:335-7. https://doi.org/10.1016/S1470-2045(20)30096-6
has received research funding from AstraZeneca, Ayala Pharmaceuticals, Bayer, Bristol-Myers Squibb, 3. Desai A, Sachdeva S, Parekh T, et al. COVID-19 and cancer: Lessons from a pooled meta-analysis. JCO
Clovis Oncology, Janssen Oncology, Merck, Pfizer (Inst), and Roche/Genentech; and has received Glob Oncol 2020;6:557-9. https://doi.org/10.1200/GO.20.00097
travel, accommodations, and expenses from Eisai Canada. Dr. Black has been advisory board member 4. Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries
or equivalent for Abbvie, Asieris, Astellas, AstraZeneca, Bayer, Biosyent, BMS, EMD-Serono, Fergene, during the incubation period of COVID-19 infection. EClinicalMedicine 2020 [Epub ahead of print].
https://doi.org/10.1016/j.eclinm.2020.100331
H3-Biomedicine, Janssen, Merck, Roche, Sanofi, and Urogen; a speakers’ bureau member for Abbvie, 5. Buriki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol 2020 [Epub ahead of print].
Biosyent, Ferring, Janssen, Pfizer, and TerSera; has received grants and/or honoraria from Bayer, https://doi.org/10.1016/S1470-2045(20)30217-5
GSK, iProgen, and Sanofi; has participated in clinical trials supported by Astellas, AstraZeneca, 6. You B, Ravaud A, Canivete A, et al. The official French guidelines to protect patients with cancer. Lancet
BMS, Genentech, Janssen, MDx Health, Pacific Edge, Sitka, and Therelase; and shares a patent Oncol 2020 [Epub ahead of print]. https://doi.org/10.1016/S1470-2045(20)30204-7
with Decipher Biosciences. Dr. Danielson has received advisory board honoraria and speaker fees 7. Lalani AA, Chi KN, Heng DYC. Prioritizing systemic therapies for genitourinary malignancies:
from Amgen, Astellas, Bayer, and Janssen. Dr. Emmenegger. Dr. Finelli has been an advisory board Canadian recommendations during the COVID-19 pandemic. Can Urol Assoc J 2020;14:E154-8.
member for Abbvie, Astellas, Bayer, Janssen, Ipsen, Sanofi, and TerSera; and has participated in https://doi.org/10.5489/cuaj.6595
clinical trials supported byAstellas, Bayer, and Janssen. Dr. Niazi has received research grants 8. NCCN, Care of prostate cancer patients during the COVID-19 pandemic: Recommendations of the NCCN.
and honoraria from Abbvie, Amgen, Astellas, Astra Zeneca, Bayer, Janssen, and Sanofi; and has Available at: https://www.nccn.org/covid-19/pdf/NCCN_PCa_COVID_guidelines.pdf. Accessed April
16, 2020.
participated in clinical trials supported by Astellas, Ferring, Janssen, and Sanofi. Dr. Pouliot has been 9. Ferreira R, McGrath M, Wang Y, et al. How to prioritize urologic surgeries during epidemics:
an advisory board member for Amgen, Astellas, Bayer, and Janssen; has received payment from Lessons learned from the Toronto SARS outbreak in 2003. Can Urol Assoc J 2020;14:E159-60.
Abbott, Amgen, Astellas, Astra Zeneca, Bayer, Ferring, Janssen, and Sanofi; has received grants https://doi.org/10.5489/cuaj.6551
from Astra Zeneca and Sanofi; and has participated in clinical trials supported by Astellas, Bayer, 10. Wilt TJ, Jones KM, Barry MJ, et al. Followup of prostatectomy versus observation for early prostate cancer.
Ferring, and Janssen. Dr. Shayegan has been an advisory board member for Astellas, Bayer, N Engl J Med 2017;377:132-42. https://doi.org/10.1056/NEJMoa1615869
and Janssen; and has received a research grant from Janssen. Dr. Sridhar has been an advisory 11. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for local-
board member for Astellas, AstraZeneca, Bayer, Janssen, Merck, and Roche; and has participated ized prostate cancer. N Engl J Med 2016;375:1415-24. https://doi.org/10.1056/NEJMoa1606220
in several pharma-supported clinical trials. Dr. Vigneault has been an advisory board member for 12. Ravi P, Karakiewicz PI, Roghmann F. Mental health outcomes in elderly men with prostate cancer. Urol
Abbvie, Bayer, Ferring, and Sanofi. Dr. Loblaw has been an advisory bpard members for Abbvie, Oncol 2014;32:1333-40. https://doi.org/10.1016/j.urolonc.2014.05.005
Amgen, Astellas, Janssen, and Sanofi; and has received honoraria from AbbVie, Astellas, Amgen, 13. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: A systematic review
AstraZeneca, Bayer, Janssen, Sanofi, and TerSera. Dr. Rendon has been an advisory board and and meta-analysis of prevalence rates. BMJ Open 2014;4:e003901. https://doi.org/10.1136/
bmjopen-2013-003901
speakers’ bureau member for, and has received honoraria from Abbvie, Amgen, Astellas, Astra 14. Rendon RA, Mason RJ, Marzouk K, et al. Canadian Urological Association recommendations on prostate
Zeneca, Bayer, Ferring, Jansen, and Sanofi. cancer screening and early diagnosis. Can Urol Assoc J 2017;11:298-309. https://doi.org/10.5489/
cuaj.4888
15. Hindson J. COVID-19: Faecal-oral transmission? Nat Rev Gastroenterol Hepatol 2020 [Epub ahead of
This consensus statement has been peer-reviewed and approved by the CUA Guidelines Committee. print]. https://doi.org/10.1038/s41575-020-0295-7
16. SAGES and EAES recommendations regarding surgical response to COVID-19 crisis. Available at: https://
www.sages.org/recommendations-surgical-response-covid-19/. Accessed April 16, 2020.
17. National Comprehensive Cancer Network. Prostate Cancer (Version 1.2020). Available at: https://www.
References nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed April 16, 2020.
18. Khan MA, Mangold LA, Epstein JI, et al. Impact of surgical delay on long-term cancer control for
clinically localized prostate cancer. J Urol 2004;172:1835-9. https://doi.org/10.1097/01.
1. Bai Y, Yao L, Wei T et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020;323:1406- ju.0000140277.08623.13
7. https://doi.org/10.1001/jama.2020.2565
CUAJ • June 2020 • Volume 14, Issue 6 167