Page 7 - Flipbook
P. 7
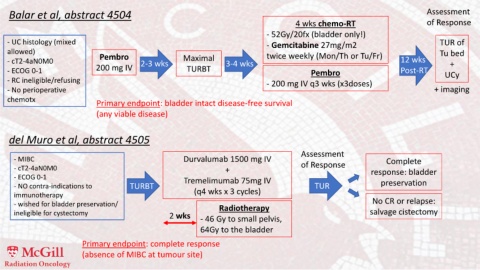
Balar et al, abstract 4504 Assessment
4 wks chemo-RT of Response
- 52Gy/20fx (bladder only!)
- UC histology (mixed - Gemcitabine 27mg/m2 TUR of
allowed) twice weekly (Mon/Th or Tu/Fr) Tu bed
- cT2-4aN0M0 Pembro 2-3 wks Maximal 3-4 wks 12 wks +
- ECOG 0-1 200 mg IV TURBT Pembro Post-RT UCy
- RC ineligible/refusing - 200 mg IV q3 wks (x3doses)
- No perioperative + imaging
chemotx
Primary endpoint: bladder intact disease-free survival
(any viable disease)
del Muro et al, abstract 4505
Assessment
- MIBC Durvalumab 1500 mg IV Complete
- cT2-4aN0M0 + of Response response: bladder
- ECOG 0-1 Tremelimumab 75mg IV
- NO contra-indications to TURBT TUR preservation
immunotherapy (q4 wks x 3 cycles)
- wished for bladder preservation/ Radiotherapy No CR or relapse:
ineligible for cystectomy 2 wks - 46 Gy to small pelvis, salvage cistectomy
64Gy to the bladder
Primary endpoint: complete response
(absence of MIBC at tumour site)
Radiation Oncology