Page 4 - BladderCancerAbstracts
P. 4
Bladder Cancer Forum 2023 abstracts
being investigated as a potential orchestrator of a local immune response. tool to better predict progression in NMIBC patients using the more widely
Mature TLS are lymph node-like structures that have an active germinal used WHO 2004/2016 grading system in North America.
center (GC) and have been associated with improved outcomes in several Methods: NIMBLE was trained on patients treated from January 2005
cancers. Here, we explored the use of TLS and their associated TME as to October 2014 at the University Health Network in Toronto (n=564).
a predictive biomarker for response to RT in MIBC. Predictors included age, sex, history of urothelial cancer, stage, grade
Methods: H&E-stained FFPE sections of pre-RT biopsies from 147 MIBC (WHO 2004/2016), concomitant carcinoma in situ (CIS), tumor burden
patients with known outcomes were examined to identify TLS presence, and size, type of intravesical therapy, European Association of Urology
with confirmation from a pathologist. For further analysis, three repre- (EAU) total progression score, and number of intermediate risk factors.
sentative tissue cores from each case were used to construct tissue micro- Internal validation was performed on patients treated from October 2014
arrays (TMA). Gene expression profiles were obtained by NanoString’s to December 2020 at the same institution (n=142). External validation
Digital Spatial technology, and immunohistochemical (IHC) staining of was performed on a publicly available dataset of patients treated from
CD20, CD4, CD8, CD68, FoxP3, and Neutrophil Elastase was performed. October 2004 to December 2013 at Seoul National University in South
Images were analyzed on the Halo platform. Korea (n=198). Primary outcome was progression, defined as relapse of
Results: H&E revealed that 19.7% of patients (n=68) had TLS with a GC, pT2 disease or higher. NIMBLE hyperparameters were tuned using a tree-
17.0% (n=25) had TLS without a GC, and 12.9% (n=19) had no TLS. In the structured Parzen estimator algorithm to optimize concordance index.
remaining 50.3% of cases (n=74), confirmation of TLS presence required NIMBLE was compared against the EAU risk groups and a previously
IHC staining, which is underway. Gene expression data showed that TLS published AI model trained on a multi-institutional European cohort.
marker CXCL13 is higher among complete responders to RT (p=0.0440). Results: Mean age of the total cohort was 68 years and 23% were female;
We also used a previously described 39-gene TLS signature to clustered 52% of patients had pTa, 43% pT1, 5% primary CIS, 42% low-grade, and
patients into two groups, “TLS high” and “TLS low.” Non-responders to 58% high-grade disease. Median followup was 4.7 years (IQR 2.2–8.3).
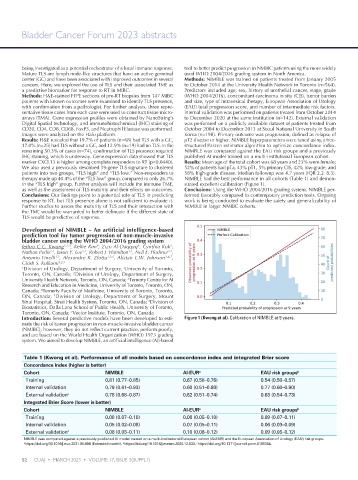
therapy made up 40.4% of the “TLS low” group, compared to only 26.7% NIMBLE had the best performance in all cohorts (Table 1) and demon-
in the “TLS high” group. Further analysis will include the immune TME, strated excellent calibration (Figure 1).
as well as the assessment of TLS maturity and their effects on outcomes. Conclusions: Using the WHO 2004/2016 grading system, NIMBLE per-
Conclusions: Our findings point to a potential role of TLS in predicting formed favorably compared to contemporary prediction tools. Ongoing
response to RT, but TLS presence alone is not sufficient to evaluate it. work is being conducted to evaluate the safety and generalizability of
Further studies to assess the maturity of TLS and their interaction with NIMBLE in larger NMIBC cohorts.
the TME would be warranted to better delineate if the different state of
TLS would be predictive of response.
Development of NIMBLE – An artificial intelligence-based
prediction tool for tumor progression of non-muscle-invasive
bladder cancer using the WHO 2004/2016 grading system
Jethro C.C. Kwong 1,2,3 , Kellie Kim , Zizo Al-Daqqaq , Cynthia Kuk ,
5
4
4
Nathan Perlis , Jason Y. Lee , Robert J. Hamilton , Neil E. Fleshner ,
1,2
1,2
1,2
1,2
Antonio Finelli , Alexandre R. Zlotta 1,2,5 , Alistair E.W. Johnson 3,6,7 ,
1,2
Girish S. Kulkarni 1,2,3
1 Division of Urology, Department of Surgery, University of Toronto,
Toronto, ON, Canada; Division of Urology, Department of Surgery,
2
University Health Network, Toronto, ON, Canada; Temerty Centre for AI
3
Research and Education in Medicine, University of Toronto, Toronto, ON,
Canada; Temerty Faculty of Medicine, University of Toronto, Toronto,
4
ON, Canada; Division of Urology, Department of Surgery, Mount
5
Sinai Hospital, Sinai Health System, Toronto, ON, Canada; Division of
6
Biostatistics, Dalla Lana School of Public Health, University of Toronto,
Toronto, ON, Canada; Vector Institute, Toronto, ON, Canada
7
Introduction: Several predictive models have been developed to esti- Figure 1 (Kwong et al). Calibration of NIMBLE at 9 years.
mate the risk of tumor progression in non-muscle-invasive bladder cancer
(NMIBC); however, they do not reflect current practice, perform poorly,
and are based on the World Health Organization (WHO) 1973 grading
system. We aimed to develop NIMBLE, an artificial intelligence (AI)-based
Table 1 (Kwong et al). Performance of all models based on concordance index and integrated Brier score
Concordance Index (higher is better)
Cohort NIMBLE AI-EUR a EAU risk groups b
Training 0.81 (0.77–0.85) 0.67 (0.58–0.76) 0.54 (0.50–0.57)
Internal validation 0.79 (0.61–0.93) 0.60 (0.51–0.80) 0.77 (0.60–0.90)
External validation c 0.78 (0.68–0.87) 0.62 (0.51–0.74) 0.63 (0.54–0.73)
Integrated Brier Score (lower is better)
Cohort NIMBLE AI-EUR a EAU risk groups b
Training 0.08 (0.07–0.10) 0.08 (0.05–0.10) 0.09 (0.07–0.11)
Internal validation 0.05 (0.02–0.08) 0.07 (0.05–0.11) 0.06 (0.03–0.09)
External validation c 0.08 (0.05–0.11) 0.10 (0.08–0.12) 0.09 (0.05–0.12)
NIMBLE was compared against a previously published AI model trained on a multi-institutional European cohort (AI-EUR) and the European Association of Urology (EAU) risk groups.
a https://doi.org/10.1016/j.euo.2021.05.006 (Extended model). b https://doi.org/10.1016/j.eururo.2020.12.033. c https://doi.org/10.1371/journal.pone.0189354.
S2 CUAJ • MARCH 2023 • VOLUME 17, ISSUE 3(SUPPL1)