Page 18 - Monitoring Prostate Cancer to Guide Treatment Decision-Making
P. 18
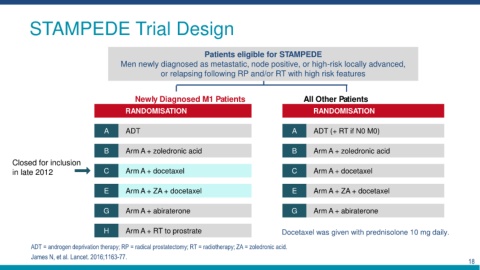
STAMPEDE Trial Design
Patients eligible for STAMPEDE
Men newly diagnosed as metastatic, node positive, or high-risk locally advanced,
or relapsing following RP and/or RT with high risk features
Newly Diagnosed M1 Patients All Other Patients
RANDOMISATION RANDOMISATION
A ADT A ADT (+ RT if N0 M0)
B Arm A + zoledronic acid B Arm A + zoledronic acid
Closed for inclusion
in late 2012 C Arm A + docetaxel C Arm A + docetaxel
E Arm A + ZA + docetaxel E Arm A + ZA + docetaxel
G Arm A + abiraterone G Arm A + abiraterone
H Arm A + RT to prostrate Docetaxel was given with prednisolone 10 mg daily.
ADT = androgen deprivation therapy; RP = radical prostatectomy; RT = radiotherapy; ZA = zoledronic acid.
James N, et al. Lancet. 2016;1163-77.
18