Page 26 - Monitoring Prostate Cancer to Guide Treatment Decision-Making
P. 26
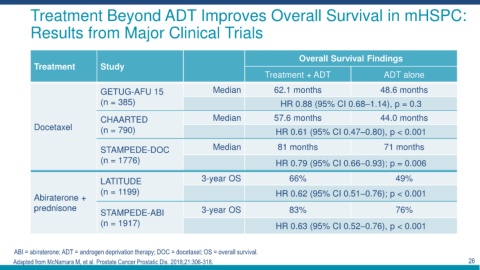
Treatment Beyond ADT Improves Overall Survival in mHSPC:
Results from Major Clinical Trials
Overall Survival Findings
Treatment Study
Treatment + ADT ADT alone
GETUG-AFU 15 Median 62.1 months 48.6 months
(n = 385) HR 0.88 (95% CI 0.68–1.14), p = 0.3
CHAARTED Median 57.6 months 44.0 months
Docetaxel (n = 790) HR 0.61 (95% CI 0.47–0.80), p < 0.001
STAMPEDE-DOC Median 81 months 71 months
(n = 1776) HR 0.79 (95% CI 0.66–0.93); p = 0.006
LATITUDE 3-year OS 66% 49%
(n = 1199) HR 0.62 (95% CI 0.51–0.76); p < 0.001
Abiraterone +
prednisone 3-year OS 83% 76%
STAMPEDE-ABI
(n = 1917) HR 0.63 (95% CI 0.52–0.76), p < 0.001
ABI = abiraterone; ADT = androgen deprivation therapy; DOC = docetaxel; OS = overall survival.
Adapted from McNamara M, et al. Prostate Cancer Prostatic Dis. 2018;21:306-318. 26