Page 25 - Management of Advanced Urothelial Carcinoma: Emerging Therapies and Biomarkers
P. 25
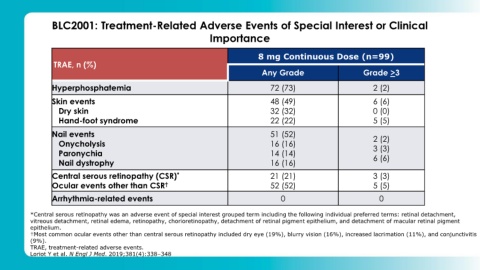
BLC2001: Treatment-Related Adverse Events of Special Interest or Clinical
Importance
8 mg Continuous Dose (n=99)
TRAE, n (%)
Any Grade Grade >3
Hyperphosphatemia 72 (73) 2 (2)
Skin events 48 (49) 6 (6)
Dry skin 32 (32) 0 (0)
Hand-foot syndrome 22 (22) 5 (5)
Nail events 51 (52)
Onycholysis 16 (16) 2 (2)
3 (3)
Paronychia 14 (14) 6 (6)
Nail dystrophy 16 (16)
Central serous retinopathy (CSR) * 21 (21) 3 (3)
Ocular events other than CSR † 52 (52) 5 (5)
Arrhythmia-related events 0 0
*Central serous retinopathy was an adverse event of special interest grouped term including the following individual preferred terms: retinal detachment,
vitreous detachment, retinal edema, retinopathy, chorioretinopathy, detachment of retinal pigment epithelium, and detachment of macular retinal pigment
epithelium.
†Most common ocular events other than central serous retinopathy included dry eye (19%), blurry vision (16%), increased lacrimation (11%), and conjunctivitis
(9%).
TRAE, treatment-related adverse events.
Loriot Y et al. N Engl J Med. 2019;381(4):338–348