Page 52 - PACE: Advanced Prostate Cancer Consensus
P. 52
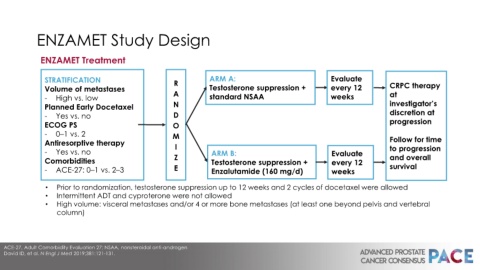
ENZAMET Study Design
ENZAMET Treatment
STRATIFICATION R ARM A: Evaluate
Volume of metastases Testosterone suppression + every 12 CRPC therapy
- High vs. low A standard NSAA weeks at
Planned Early Docetaxel N investigator’s
- Yes vs. no D discretion at
ECOG PS O progression
- 0–1 vs. 2 M
Antiresorptive therapy I Follow for time
- Yes vs. no ARM B: Evaluate to progression
Comorbidities Z Testosterone suppression + every 12 and overall
- ACE-27: 0–1 vs. 2–3 E Enzalutamide (160 mg/d) weeks survival
• Prior to randomization, testosterone suppression up to 12 weeks and 2 cycles of docetaxel were allowed
• Intermittent ADT and cyproterone were not allowed
• High volume: visceral metastases and/or 4 or more bone metastases (at least one beyond pelvis and vertebral
column)
ACE-27, Adult Comorbidity Evaluation 27; NSAA, nonsteroidal anti-androgen
David ID, et al. N Engl J Med 2019;381:121-131.