Page 106 - Flipbook
P. 106
Adverse Events (AEs) and Antidrug Antibodies (ADAs)
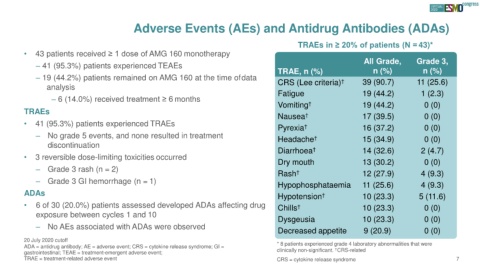
TRAEs in ≥ 20% of patients (N = 43)*
• 43 patients received ≥ 1 dose of AMG 160 monotherapy
All Grade, Grade 3,
– 41 (95.3%) patients experienced TEAEs
TRAE, n (%) n (%) n (%)
– 19 (44.2%) patients remained on AMG 160 at the time ofdata † 39 (90.7) 11 (25.6)
analysis CRS (Lee criteria)
Fatigue 19 (44.2) 1 (2.3)
– 6 (14.0%) received treatment ≥ 6 months
Vomiting † 19 (44.2) 0 (0)
TRAEs
Nausea † 17 (39.5) 0 (0)
• 41 (95.3%) patients experienced TRAEs
Pyrexia † 16 (37.2) 0 (0)
– No grade 5 events, and none resulted in treatment Headache † 15 (34.9) 0 (0)
discontinuation
Diarrhoea † 14 (32.6) 2 (4.7)
• 3 reversible dose-limiting toxicities occurred
Dry mouth 13 (30.2) 0 (0)
– Grade 3 rash (n = 2)
Rash † 12 (27.9) 4 (9.3)
– Grade 3 GI hemorrhage (n = 1) Hypophosphataemia 11 (25.6) 4 (9.3)
ADAs Hypotension † 10 (23.3) 5 (11.6)
• 6 of 30 (20.0%) patients assessed developed ADAs affecting drug Chills † 10 (23.3) 0 (0)
exposure between cycles 1 and 10
Dysgeusia 10 (23.3) 0 (0)
– No AEs associated with ADAs were observed
Decreased appetite 9 (20.9) 0 (0)
20 July 2020 cutoff
ADA = antidrug antibody; AE = adverse event; CRS = cytokine release syndrome; GI = * 8 patients experienced grade 4 laboratory abnormalities that were
†
gastrointestinal; TEAE = treatment-emergent adverse event; clinically non-significant. CRS-related
TRAE = treatment-related adverse event CRS = cytokine release syndrome 7