Page 51 - Flipbook
P. 51
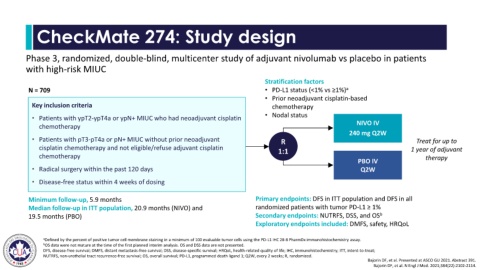
CheckMate 274: Study design
Phase 3, randomized, double-blind, multicenter study of adjuvant nivolumab vs placebo in patients
with high-risk MIUC
Stratification factors
N = 709 • PD-L1 status (<1% vs ≥1%) a
• Prior neoadjuvant cisplatin-based
Key inclusion criteria chemotherapy
• Nodal status
• Patients with ypT2-ypT4a or ypN+ MIUC who had neoadjuvant cisplatin
chemotherapy NIVO IV
240 mg Q2W
• Patients with pT3-pT4a or pN+ MIUC without prior neoadjuvant R Treat for up to
cisplatin chemotherapy and not eligible/refuse adjuvant cisplatin 1:1 1 year of adjuvant
chemotherapy therapy
PBO IV
• Radical surgery within the past 120 days Q2W
• Disease-free status within 4 weeks of dosing
Minimum follow-up, 5.9 months Primary endpoints: DFS in ITT population and DFS in all
Median follow-up in ITT population, 20.9 months (NIVO) and randomized patients with tumor PD-L1 ≥ 1%
19.5 months (PBO) Secondary endpoints: NUTRFS, DSS, and OS b
Exploratory endpoints included: DMFS, safety, HRQoL
a Defined by the percent of positive tumor cell membrane staining in a minimum of 100 evaluable tumor cells using the PD-L1 IHC 28-8 PharmDx immunohistochemistry assay.
b OS data were not mature at the time of the first planned interim analysis. OS and DSS data are not presented.
DFS, disease-free survival; DMFS, distant metastasis-free survival; DSS, disease-specific survival; HRQoL, health-related quality of life; IHC, immunohistochemistry; ITT, intent-to-treat;
NUTRFS, non-urothelial tract recurrence-free survival; OS, overall survival; PD-L1, programmed death ligand 1; Q2W, every 2 weeks; R, randomized.
Bajorin DF, et al. Presented at ASCO GU 2021. Abstract 391.
Bajorin DF, et al. N Engl J Med. 2021;384(22):2102-2114.