Page 52 - Flipbook
P. 52
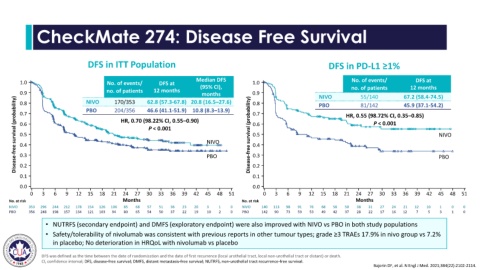
CheckMate 274: Disease Free Survival
DFS in ITT Population DFS in PD-L1 ≥1%
Median DFS No. of events/ DFS at
1.0 No. of events/ DFS at 1.0
(95% CI), no. of patients 12 months
no. of patients 12 months
0.9 NIVO 170/353 62.8 (57.3-67.8) 20.8 (16.5–27.6) 0.9 NIVO 55/140 67.2 (58.4-74.5)
months
Disease-free survival (probability) 0.6 HR, 0.70 (98.22% CI, 0.55–0.90) NIVO Disease-free survival (probability) 0.7 HR, 0.55 (98.72% CI, 0.35–0.85) NIVO
0.8
0.8
81/142
PBO
45.9 (37.1-54.2)
46.6 (41.1-51.9) 10.8 (8.3–13.9)
204/356
PBO
0.7
P < 0.001
0.6
P < 0.001
0.5
0.5
0.4
0.4
0.3
0.3
0.2
0.1
0.1 PBO 0.2 PBO
0.0 0.0
0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51
No. at risk Months No. at risk Months
NIVO 353 296 244 212 178 154 126 106 85 68 57 51 36 23 20 3 1 0 NIVO 140 113 98 91 76 68 58 50 38 31 27 24 21 12 10 1 0 0
PBO 356 248 198 157 134 121 103 94 80 65 54 50 37 22 19 10 2 0 PBO 142 90 73 59 53 49 42 37 28 22 17 16 12 7 5 3 1 0
• NUTRFS (secondary endpoint) and DMFS (exploratory endpoint) were also improved with NIVO vs PBO in both study populations
• Safety/tolerability of nivolumab was consistent with previous reports in other tumour types; grade ≥3 TRAEs 17.9% in nivo group vs 7.2%
in placebo; No deterioration in HRQoL with nivolumab vs placebo
DFS was defined as the time between the date of randomization and the date of first recurrence (local urothelial tract, local non-urothelial tract or distant) or death.
CI, confidence interval; DFS, disease-free survival; DMFS, distant metastasis-free survival; NUTRFS, non-urothelial tract recurrence-free survival.
Bajorin DF, et al. N Engl J Med. 2021;384(22):2102-2114.