Page 39 - Flipbook
P. 39
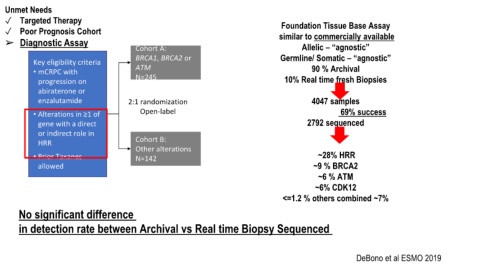
Unmet Needs
✓ Targeted Therapy Foundation Tissue Base Assay
✓ Poor Prognosis Cohort similar to commercially available
➢ Diagnostic Assay
Cohort A: Allelic – “agnostic”
BRCA1, BRCA2 or Germline/ Somatic – “agnostic”
Key eligibility criteria
• mCRPC with ATM 90 % Archival
N=245 10% Real time fresh Biopsies
progression on
abiraterone or
enzalutamide 2:1 randomization 4047 samples
• Alterations in ≥1 of Open-label 69% success
gene with a direct 2792 sequenced
or indirect role in
HRR Cohort B:
Other alterations
• Prior Taxanes N=142 ~28% HRR
allowed ~9 % BRCA2
~6 % ATM
~6% CDK12
<=1.2 % others combined ~7%
No significant difference
in detection rate between Archival vs Real time Biopsy Sequenced
DeBono et al ESMO 2019