Page 34 - Flipbook
P. 34
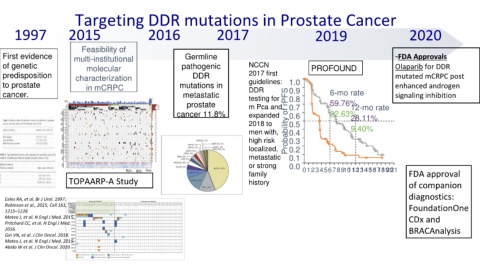
Targeting DDR mutations in Prostate Cancer
1997 2015 2016 2017 2019 2020
Feasibility of
First evidence multi-institutional Germline -FDA Approvals
of genetic molecular pathogenic NCCN PROFOUND Olaparib for DDR
predisposition characterization DDR 2017 first mutated mCRPC post
to prostate in mCRPC mutations in guidelines: 1.0 enhanced androgen
cancer. metastatic DDR 0.9 6-mo rate signaling inhibition
testing for
0.8
prostate m Pca and 0.7 59.76%
12-mo rate
cancer 11.8% expanded 0.6 22.63%
28.11%
2018 to Probability of rPFS 0.5
men with, 0.4 9.40%
high risk 0.3
localized, 0.2
metastatic 0.1
or strong 0.0
family 0 123456789101112131415161718192021 FDA approval
TOPAARP-A Study history of companion
diagnostics:
Eeles RA, et al. Br J Urol. 1997.
Robinson et al., 2015, Cell 161, FoundationOne
1215–1228
Mateo J, et al. N Engl J Med. 2015 CDx and
Pritchard CC, et al. N Engl J Med.
2016. BRACAnalysis
Giri VN, et al. J Clin Oncol. 2018.
Mateo J, et al. N Engl J Med. 2015.
Abida W et al. J Clin Oncol. 2020