Page 10 - Flipbook
P. 10
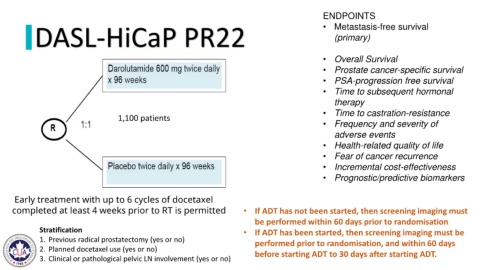
ENDPOINTS
DASL-HiCaP PR22 • Metastasis-free survival
(primary)
• Overall Survival
• Prostate cancer-specific survival
• PSA-progression free survival
• Time to subsequent hormonal
therapy
• Time to castration-resistance
1,100 patients
• Frequency and severity of
adverse events
• Health-related quality of life
• Fear of cancer recurrence
• Incremental cost-effectiveness
• Prognostic/predictive biomarkers
Early treatment with up to 6 cycles of docetaxel
completed at least 4 weeks prior to RT is permitted • If ADT has not been started, then screening imaging must
be performed within 60 days prior to randomisation
Stratification • If ADT has been started, then screening imaging must be
1. Previous radical prostatectomy (yes or no) performed prior to randomisation, and within 60 days
2. Planned docetaxel use (yes or no)
before starting ADT to 30 days after starting ADT.
3. Clinical or pathological pelvic LN involvement (yes or no)