Page 15 - Flipbook
P. 15
15
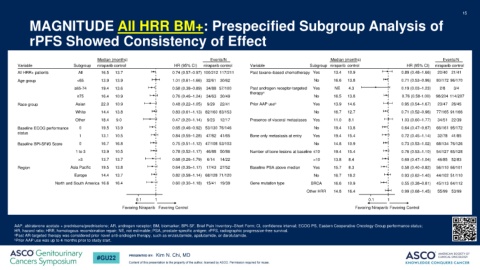
MAGNITUDE All HRR BM+: Prespecified Subgroup Analysis of
rPFS Showed Consistency of Effect
Median (months) Events/N Median (months) Events/N
Variable Subgroup niraparib control HR (95% CI) niraparib control Variable Subgroup niraparib control HR (95% CI) niraparib control
All HRR+ patients All 16.5 13.7 0.74 (0.57–0.97) 100/212 117/211 Past taxane–based chemotherapy Yes 13.4 10.9 0.89 (0.48–1.66) 20/40 21/41
Age group <65 13.9 13.9 1.01 (0.61–1.66) 32/61 30/62 No 16.6 13.8 0.71 (0.53–0.96) 80/172 96/170
≥65-74 19.4 13.6 0.58 (0.38–0.89) 34/88 57/100 Past androgen receptor-targeted Yes NE 4.3 0.19 (0.03–1.23) 2/8 3/4
therapy a
≥75 16.4 10.9 0.76 (0.46–1.24) 34/63 30/49 No 16.5 13.8 0.76 (0.58-1.00) 98/204 114/207
Race group Asian 22.0 10.9 0.48 (0.22–1.05) 9/29 22/41 Prior AAP use b Yes 13.9 14.6 0.95 (0.54–1.67) 23/47 26/45
White 14.4 13.8 0.83 (0.61–1.13) 82/160 83/153 No 16.7 12.7 0.71 (0.52–0.96) 77/165 91/166
Other 18.4 9.0 0.47 (0.20–1.14) 9/23 12/17 Presence of visceral metastases Yes 11.0 8.1 1.03 (0.60–1.77) 34/51 22/39
Baseline ECOG performance 0 19.5 13.9 0.65 (0.46–0.92) 53/130 76/146 No 19.4 13.8 0.64 (0.47–0.87) 66/161 95/172
status
1 13.1 10.5 0.84 (0.55–1.28) 47/82 41/65 Bone only metastasis at entry Yes 19.4 15.4 0.72 (0.45–1.14) 32/78 41/85
Baseline BPI-SF#3 Score 0 16.7 16.8 0.75 (0.51–1.12) 47/108 53/103 No 14.8 10.9 0.73 (0.53–1.02) 68/134 76/126
1 to 3 13.9 10.5 0.78 (0.52–1.17) 46/88 50/86 Number of bone lesions at baseline ≤10 19.4 15.4 0.76 (0.53–1.10) 54/127 65/128
>3 13.7 13.7 0.68 (0.26–1.79) 6/14 14/22 >10 13.8 8.4 0.69 (0.47–1.04) 46/85 52/83
Region Asia Pacific 19.5 13.8 0.64 (0.35–1.17) 17/43 27/52 Baseline PSA above median Yes 15.7 8.3 0.58 (0.40–0.82) 56/110 66/101
Europe 14.4 13.7 0.82 (0.58–1.14) 68/128 71/120 No 16.7 18.2 0.93 (0.62–1.40) 44/102 51/110
North and South America 16.6 16.4 0.60 (0.30–1.18) 15/41 19/39 Gene mutation type BRCA 16.6 10.9 0.55 (0.38–0.81) 45/113 64/112
Other HRR 14.8 16.4 0.99 (0.68–1.45) 55/99 53/99
0.1 1 0.1 1
Favoring Niraparib Favoring Control Favoring Niraparib Favoring Control
AAP, abiraterone acetate + prednisone/prednisolone; AR, androgen receptor; BM, biomarker; BPI-SF, Brief Pain Inventory–Short Form; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status;
HR, hazard ratio; HRR, homologous recombination repair; NE, not estimable; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival.
a Past AR-targeted therapy was considered prior novel anti-androgen therapy, such as enzalutamide, apalutamide, or darolutamide.
b Prior AAP use was up to 4 months prior to study start.
PRESENTED BY: Kim N. Chi, MD