Page 12 - Flipbook
P. 12
12
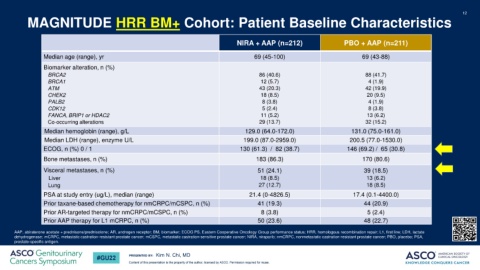
MAGNITUDE HRR BM+ Cohort: Patient Baseline Characteristics
NIRA + AAP (n=212) PBO + AAP (n=211)
Median age (range), yr 69 (45-100) 69 (43-88)
Biomarker alteration, n (%)
BRCA2 86 (40.6) 88 (41.7)
BRCA1 12 (5.7) 4 (1.9)
ATM 43 (20.3) 42 (19.9)
CHEK2 18 (8.5) 20 (9.5)
PALB2 8 (3.8) 4 (1.9)
CDK12 5 (2.4) 8 (3.8)
FANCA, BRIP1 or HDAC2 11 (5.2) 13 (6.2)
Co-occurring alterations 29 (13.7) 32 (15.2)
Median hemoglobin (range), g/L 129.0 (64.0-172.0) 131.0 (75.0-161.0)
Median LDH (range), enzyme U/L 199.0 (87.0-2959.0) 200.5 (77.0-1530.0)
ECOG, n (%) 0 / 1 130 (61.3) / 82 (38.7) 146 (69.2) / 65 (30.8)
Bone metastases, n (%) 183 (86.3) 170 (80.6)
Visceral metastases, n (%) 51 (24.1) 39 (18.5)
Liver 18 (8.5) 13 (6.2)
Lung 27 (12.7) 18 (8.5)
PSA at study entry (ug/L), median (range) 21.4 (0-4826.5) 17.4 (0.1-4400.0)
Prior taxane-based chemotherapy for nmCRPC/mCSPC, n (%) 41 (19.3) 44 (20.9)
Prior AR-targeted therapy for nmCRPC/mCSPC, n (%) 8 (3.8) 5 (2.4)
Prior AAP therapy for L1 mCRPC, n (%) 50 (23.6) 48 (22.7)
AAP, abiraterone acetate + prednisone/prednisolone; AR, androgen receptor; BM, biomarker; ECOG PS, Eastern Cooperative Oncology Group performance status; HRR, homologous recombination repair; L1, first line; LDH, lactate
dehydrogenase; mCRPC, metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; NIRA, niraparib; nmCRPC, nonmetastatic castration-resistant prostate cancer; PBO, placebo; PSA,
prostate-specific antigen.
PRESENTED BY: Kim N. Chi, MD