Page 9 - Flipbook
P. 9
MAGNITUDE: Randomized, Double-Blind, Placebo-Controlled Study 9
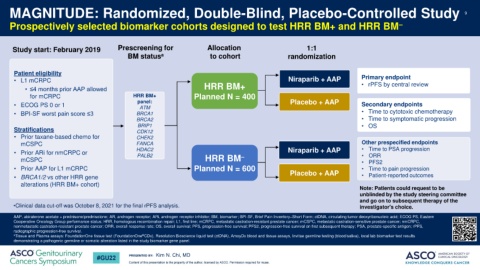
Prospectively selected biomarker cohorts designed to test HRR BM+ and HRR BM –
Study start: February 2019 Prescreening for Allocation 1:1
BM status a to cohort randomization
Patient eligibility
• L1 mCRPC Niraparib + AAP Primary endpoint
• rPFS by central review
• ≤4 months prior AAP allowed HRR BM+
for mCRPC HRR BM+ Planned N = 400
panel: Placebo + AAP
• ECOG PS 0 or 1 ATM Secondary endpoints
• BPI-SF worst pain score ≤3 BRCA1 • Time to cytotoxic chemotherapy
BRCA2 • Time to symptomatic progression
BRIP1 • OS
Stratifications CDK12
• Prior taxane-based chemo for CHEK2
mCSPC FANCA Other prespecified endpoints
HDAC2 Niraparib + AAP • Time to PSA progression
• Prior ARi for nmCRPC or PALB2 • ORR
mCSPC HRR BM – • PFS2
• Prior AAP for L1 mCRPC Planned N = 600 Placebo + AAP • Time to pain progression
• BRCA1/2 vs other HRR gene • Patient-reported outcomes
alterations (HRR BM+ cohort)
Note: Patients could request to be
unblinded by the study steering committee
and go on to subsequent therapy of the
•Clinical data cut-off was October 8, 2021 for the final rPFS analysis. investigator's choice.
AAP, abiraterone acetate + prednisone/prednisolone; AR, androgen receptor; ARi, androgen receptor inhibitor; BM, biomarker; BPI-SF, Brief Pain Inventory–Short Form; ctDNA, circulating tumor deoxyribonucleic acid; ECOG PS, Eastern
Cooperative Oncology Group performance status; HRR, homologous recombination repair; L1, first line; mCRPC, metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; nmCRPC,
nonmetastatic castration-resistant prostate cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PFS2, progression-free survival on first subsequent therapy; PSA, prostate-specific antigen; rPFS,
radiographic progression-free survival.
a Tissue and Plasma assays: FoundationOne tissue test (FoundationOne CDx), Resolution Bioscience liquid test (ctDNA), AmoyDx blood and tissue assays, Invitae germline testing (blood/saliva), local lab biomarker test results
®
demonstrating a pathogenic germline or somatic alteration listed in the study biomarker gene panel.
PRESENTED BY: Kim N. Chi, MD