Page 17 - Kidney Cancer Research Network of Canada (KCRNC) consensus statement on the role of cytoreductive nephrectomy for patients with metastatic renal cell carcinoma
P. 17
CUAJ – Consensus Statement Mason et al
KCRNC consensus: Cytoreductive nephrectomy for mRCC
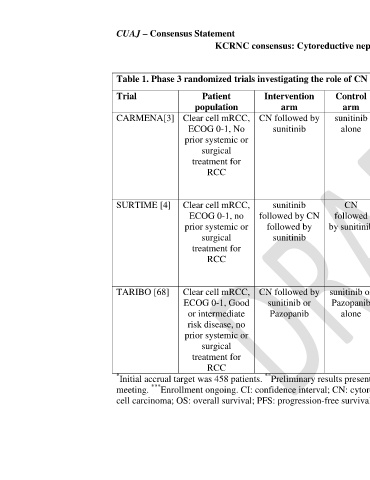
Table 1. Phase 3 randomized trials investigating the role of CN in patients with mRCC in the targeted therapy era
Trial Patient Intervention Control Outcomes Sample Median Results for primary
population arm arm size followup outcome
CARMENA[3] Clear cell mRCC, CN followed by sunitinib Primary – OS n=452 50.2 HR for OS:
ECOG 0-1, No sunitinib alone Secondary – months 0.89 (95% CI, 0.71 to
prior systemic or Objective 1.10)
surgical response, PFS,
treatment for Treatment
RCC compliance,
Safety and
adverse events
*
SURTIME [4] Clear cell mRCC, sunitinib CN Primary – Disease n=99 30.9 Progression at 28
**
ECOG 0-1, no followed by CN followed progression at 28 months weeks :
prior systemic or followed by by sunitinib weeks Upfront CN - 42.0%
surgical sunitinib Secondary – OS, Upfront sunitinib –
treatment for Objective 42.9%
RCC response, Safety p>0.99
and adverse
events
TARIBO [68] Clear cell mRCC, CN followed by sunitinib or Primary – OS n=270 *** ***
ECOG 0-1, Good sunitinib or Pazopanib Secondary – (estimated)
or intermediate Pazopanib alone Objective
risk disease, no response, PFS,
prior systemic or Safety and
surgical adverse events,
treatment for Biomarker
RCC analysis
* Initial accrual target was 458 patients. Preliminary results presented at the 2017 European Society of Medical Oncology annual
**
meeting. *** Enrollment ongoing. CI: confidence interval; CN: cytoreductive nephrectomy; HR: hazard ratio; mRCC: metastatic renal
cell carcinoma; OS: overall survival; PFS: progression-free survival.