Page 14 - CUA 2020_Functional Urology
P. 14
Unmoderated Posters 3: Prostate Cancer, Functional Urology, Other Urology Topics
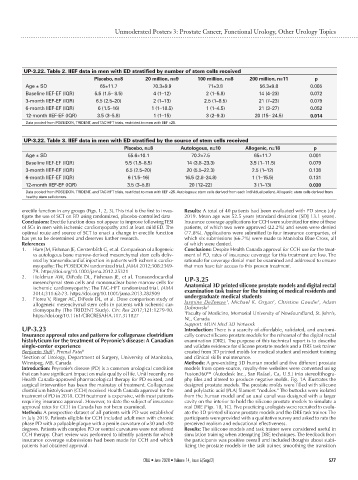
UP-3.22. Table 2. IIEF data in men with ED stratified by number of stem cells received
Placebo, n=8 20 million, n=9 100 million, n=8 200 million, n=11 p
Age ± SD 65±11.7 70.3±9.9 71±3.8 56.3±9.8 0.005
Baseline IIEF-EF (IQR) 5.5 (1.5– 8.5) 4 (1–12) 2 (1–5.8) 14 (4–23) 0.072
3-month IIEF-EF (IQR) 6.5 (2.5–20) 2 (1–13) 2.5 (1–8.5) 21 (7–23) 0.079
6-month IIEF-EF (IQR) 6 (1.5–16) 1 (1–18.5) 1 (1–4.5) 21 (3–27) 0.052
12-month IIEF-EF (IQR) 3.5 (3–5.8) 1 (1–15) 3 (2–9.3) 20 (15– 24.5) 0.014
Data pooled from POSEIDON, TRIDENT, and TAC-HFT trials, restricted to men with IIEF <25.
UP-3.22. Table 3. IIEF data in men with ED stratified by the source of stem cells received
Placebo, n=8 Autologous, n=10 Allogenic, n=18 p
Age ± SD 55.6±10.1 70.2±7.5 65±11.7 0.001
Baseline IIEF-EF (IQR) 5.5 (1.5–8.5) 14 (3.8–23.3) 3.5 (1–11.5) 0.079
3-month IIEF-EF (IQR) 6.5 (2.5–20) 20 (5.5–22.3) 2.5 (1–12) 0.138
6-month IIEF-EF (IQR) 6 (1.5–16) 16.5 (2.8–24.8) 1 (1–15.5) 0.131
12-month IIEF-EF (IQR) 3.5 (3–5.8) 20 (12–22) 3 (1–13) 0.030
Data pooled from POSEIDON, TRIDENT, and TAC-HFT trials, restricted to men with IIEF <25. Autologous: stem cells derived from each individual patient. Allogenic: stem cells derived from
healthy stem cell donors.
erectile function in any groups (Figs. 1, 2, 3). This trial is the first to inves- Results: A total of 40 patients had been evaluated with PD since July
tigate the use of SCT on ED using randomized, placebo-controlled data 2019. Mean age was 52.5 years (standard deviation [SD] 13.1 years).
Conclusions: Erectile function does not appear to improve following TESI Insurance coverage applications for CCH were submitted for nine of these
of SCs in men with ischemic cardiomyopathy and at least mild ED. The patients, of which two were approved (22.2%) and seven were denied
optimal route and source of SCT to enact a change in erectile function (77.8%). Applications were submitted to four insurance companies, of
has yet to be determined and deserves further research. which six submissions (66.7%) were made to Manitoba Blue Cross, all
References of which were denied.
1. Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic Conclusions: Despite Health Canada approval for CCH use for the treat-
vs autologous bone marrow-derived mesenchymal stem cells deliv- ment of PD, rates of insurance coverage for this treatment are low. The
ered by transendocardial injection in patients with ischemic cardio- rationale for coverage denial must be examined and addressed to ensure
myopathy: The POSEIDON randomized trial. JAMA 2012;308:2369- that men have fair access to this proven treatment.
79. https://doi.org/10.1001/jama.2012.25321
2. Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial UP-3.25
mesenchymal stem cells and mononuclear bone marrow cells for Anatomical 3D printed silicone prostate models and digital rectal
ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA examination task trainer for the training of medical residents and
2014;311:62-73. https://doi.org/10.1001/jama.2013.282909 undergraduate medical students
3. Florea V, Rieger AC, DiFede DL, et al. Dose comparison study of 1 1 1
allogeneic mesenchymal stem cells in patients with ischemic car- Jasmine DeZeeuw , Michael K. Organ , Christine Goudie , Adam
1
diomyopathy (The TRIDENT Study). Circ Res 2017;121:1279-90. Dubrowski
1
https://doi.org/10.1161/CIRCRESAHA.117.311827 Faculty of Medicine, Memorial University of Newfoundland, St. John’s,
NL, Canada
Support: MUN Med 3D Network
UP-3.23 Introduction: There is a scarcity of affordable, validated, and anatomi-
Insurance approval rates and patterns for collagenase clostridium cally correct silicone prostate models for the rehearsal of the digital rectal
histolyticum for the treatment of Peyronie’s disease: A Canadian examination (DRE). The purpose of this technical report is to describe
single-center experience and validate evidence for silicone prostate models and a DRE task trainer
1
Benjamin Shiff , Premal Patel 1 created from 3D printed molds for medical student and resident training
1 Section of Urology, Department of Surgery, University of Manitoba, and clinical skills maintenance.
Winnipeg, MB, Canada Methods: A pre-existing 3D human model and five different prostate
Introduction: Peyronie’s disease (PD) is a common urological condition models from open-source, royalty-free websites were converted using
that can have significant impact on male quality of life. Until recently, no Fusion360™ (Autodesk Inc., San Rafael, Ca, U.S.) into stereolithogra-
Health Canada-approved pharmacological therapy for PD existed, and phy files and altered to produce negative molds. Fig. 1A illustrates the
surgical intervention has been the mainstay of treatment. Collagenase designed prostate models. The prostate molds were filled with silicone
clostridium histolyticum (CCH) received Health Canada approval for the and polylactic acid (PLA) filament “nodules.” The buttocks were isolated
treatment of PD in 2018. CCH treatment is expensive, with most patients from the human model and an anal canal was designed with a larger
requiring insurance approval. However, to date the subject of insurance cavity on the interior to hold the silicone prostate models to simulate a
approval rates for CCH in Canada has not been examined. real DRE (Figs. 1B, 1C). Five practicing urologists were recruited to evalu-
Methods: A prospective dataset of all patients with PD was established ate the 3D printed silicone prostate models and the DRE task trainer. The
in July 2019. Patients eligible for CCH included adult men with chronic participants were provided with a qualitative survey and asked to rate the
phase PD with a palpable plaque with a penile curvature of ≥30 and <90 perceived realism and educational effectiveness.
degrees. Patients with complex PD or ventral curvatures were not offered Results: The silicone models and task trainer were considered useful in
CCH therapy. Chart review was performed to identify patients for which simulation training when attempting DRE techniques. The feedback from
insurance coverage submissions had been made for CCH and which the participants was positive overall and included thoughts about stabi-
patients had obtained approval. lizing the prostate models in the task trainer, smoothing the transition
CUAJ • June 2020 • Volume 14, Issue 6(Suppl2) S77