Page 5 - CUA Adv Prostate Ca Drug Access List-MAR-2022
P. 5
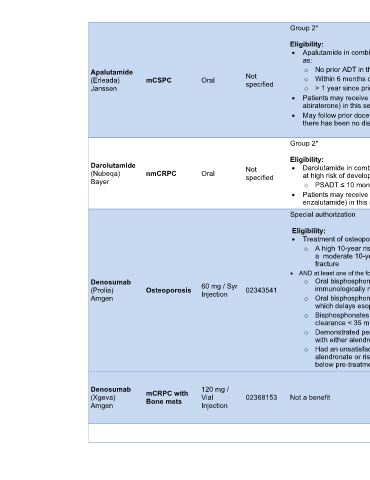
AB
Group 2* AHS Outpatient
Cancer Drug
Eligibility: Benefit Program
• Apalutamide in combination with ADT for the treatment of patients with mCSPC defined [3-22]
as:
Apalutamide o No prior ADT in the metastatic setting, OR
(Erleada) mCSPC Oral Not o Within 6 months of beginning ADT for metastatic disease, OR
specified
Janssen o > 1 year since prior ADT for early-stage disease with good performance status
• Patients may receive only one of these agents (apalutamide, enzalutamide, or
abiraterone) in this setting and switching only if intolerance, not progression
• May follow prior docetaxel for mCSPC, if treatment has been within the last 3 months and
there has been no disease progression (time limited needed)
Group 2* AHS Outpatient
Cancer Drug
Eligibility: Benefit Program
Darolutamide Not • Darolutamide in combination with ADT for the treatment of patients with nmCRPC who are [3-22]
(Nubeqa) nmCRPC Oral specified at high risk of developing metastases. High risk defined as:
Bayer o PSADT ≤ 10 months during continuous ADT/post orchiectomy
• Patients may receive only one of these agents (darolutamide, apalutamide or
enzalutamide) in this setting and switching only if intolerant (without progression)
Special authorization Alberta Health
Drug Benefit List
Eligibility: [3-22]
• Treatment of osteoporosis in patients who have:
o A high 10-year risk (> 20%) of experiencing a major osteoporotic fracture OR
a moderate 10-year fracture risk (10-20%) and have experienced a prior fragility
fracture
• AND at least one of the following:
Denosumab 60 mg / Syr o Oral bisphosphonates are contraindicated due to drug-induced hypersensitivity (ie,
(Prolia) Osteoporosis Injection 02343541 immunologically mediated), OR
Amgen o Oral bisphosphonates are contraindicated due to an abnormality of the esophagus
which delays esophageal emptying, OR
o Bisphosphonates are contraindicated due to severe renal impairment (i.e. creatinine
clearance < 35 mL/min), OR
o Demonstrated persistent severe gastrointestinal intolerance to a course of therapy
with either alendronate or risedronate, OR
o Had an unsatisfactory response (defined as a fragility fracture despite adhering to oral
alendronate or risedronate treatment fully for 1 year and evidence of a decline in BMD
below pre-treatment baseline level).
Alberta Health
Drug Benefit List
Denosumab mCRPC with 120 mg / [3-22]
(Xgeva) Bone mets Vial 02368153 Not a benefit
Amgen Injection
Page 3 | © Canadian Urological Association
v.01-MAR-2022