Page 7 - CUA Adv Prostate Ca Drug Access List-MAR-2022
P. 7
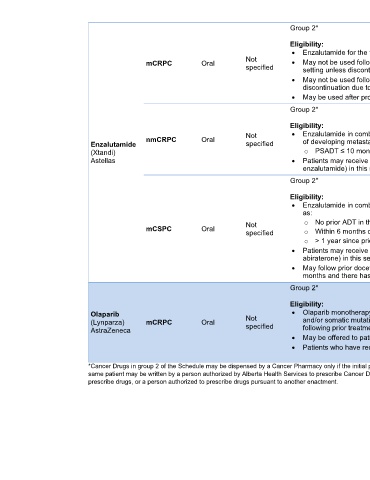
AB
Group 2* AHS Outpatient
Cancer Drug
Eligibility: Benefit Program
• Enzalutamide for the treatment of mCRPC [3-22]
mCRPC Oral Not • May not be used following apalutamide, enzalutamide, or darolutamide use in nmCRPC
specified setting unless discontinuation due to intolerance (without progression)
• May not be used following apalutamide or enzalutamide use in mCSPC setting unless
discontinuation due to intolerance (without progression)
• May be used after progression on docetaxel if not received before
Group 2* AHS Outpatient
Cancer Drug
Eligibility: Benefit Program
nmCRPC Oral Not • Enzalutamide in combination with ADT for the treatment of nmCRPC who are at high risk [3-22]
Enzalutamide specified of developing metastases. High risk defined as:
(Xtandi) o PSADT ≤ 10 months during continuous ADT/post orchiectomy
Astellas • Patients may receive only one of these agents (darolutamide, apalutamide or
enzalutamide) in this setting and switching only if intolerant (without progression)
Group 2* AHS Outpatient
Cancer Drug
Eligibility: Benefit Program
• Enzalutamide in combination with ADT for the treatment of patients with mCSPC defined [3-22]
as:
mCSPC Oral Not o No prior ADT in the metastatic setting, OR
o Within 6 months of beginning ADT, OR
specified
o > 1 year since prior ADT for early-stage disease with good performance status
• Patients may receive only one of these agents (apalutamide, enzalutamide, or
abiraterone) in this setting and switching only if intolerant (without progression)
• May follow prior docetaxel for mCSPC provided treatment has been within the last 3
months and there has been no disease progression (time limited need)
Group 2* AHS Outpatient
Cancer Drug
Eligibility: Benefit Program
Olaparib Not • Olaparib monotherapy for mCRPC and deleterious or suspected deleterious germline [3-22]
(Lynparza) mCRPC Oral specified and/or somatic mutations in the HRR genes BRCA1/2 or ATM who have progressed
AstraZeneca following prior treatment with ARAT
• May be offered to patients who are unable to tolerate an ARAT
• Patients who have received prior taxane-based chemotherapy are eligible
*Cancer Drugs in group 2 of the Schedule may be dispensed by a Cancer Pharmacy only if the initial prescription is written by a Cancer Centre Medical Staff member, but a subsequent prescription for the
same patient may be written by a person authorized by Alberta Health Services to prescribe Cancer Drugs and who is a physician, a regulated member under the Health Professions Act authorized to
prescribe drugs, or a person authorized to prescribe drugs pursuant to another enactment.
Page 4 | © Canadian Urological Association
v.01-MAR-2022