Page 9 - Flipbook
P. 9
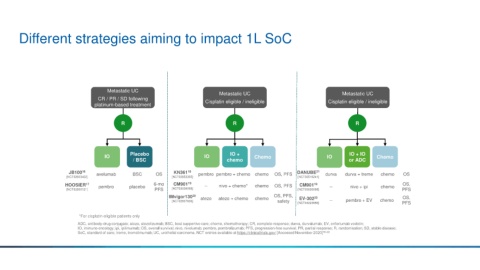
Different strategies aiming to impact 1L SoC
Metastatic UC
Metastatic UC Metastatic UC
CR / PR / SD following Cisplatin eligible / ineligible Cisplatin eligible / ineligible
platinum-based treatment
R R R
Placebo IO + IO + IO
IO IO Chemo IO Chemo
/ BSC chemo or ADC
JB100 16 avelumab BSC OS KN361 18 pembro pembro + chemo chemo OS, PFS DANUBE 21 durva durva + treme chemo OS
[NCT02603432] [NCT02853305] [NCT02516241]
HOOSIER 17 pembro placebo 6-mo CM901 19 -- nivo + chemo* chemo OS, PFS CM901 19 -- nivo + ipi chemo OS,
[NCT02500121] PFS [NCT03036098] [NCT03036098] PFS
IMvigor130 20 atezo atezo + chemo chemo OS, PFS, EV-302 22 OS,
[NCT02807636] safety -- pembro + EV chemo
[NCT04223856] PFS
*For cisplatin-eligible patients only
ADC, antibody-drug conjugate; atezo, atezolizumab; BSC, best supportive care; chemo, chemotherapy; CR, complete response; durva, durvalumab; EV, enfortumab vedotin;
IO, immuno-oncology; ipi, ipilimumab; OS, overall survival; nivo, nivolumab; pembro, pembrolizumab; PFS, progression-free survival; PR, partial response; R, randomisation; SD, stable disease;
SoC, standard of care; treme, tremelimumab; UC, urothelial carcinoma. NCT entries available at https://clinicaltrials.gov/ [Accessed November 2020] 16–22