Page 7 - Flipbook
P. 7
BPR: Catheter use
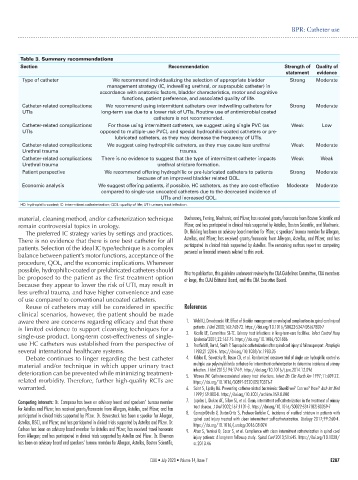
Table 3. Summary recommendations
Section Recommendation Strength of Quality of
statement evidence
Type of catheter We recommend individualizing the selection of appropriate bladder Strong Moderate
management strategy (IC, indwelling urethral, or suprapubic catheter) in
accordance with anatomic factors, bladder characteristics, motor and cognitive
functions, patient preference, and associated quality of life.
Catheter-related complications: We recommend using intermittent catheters over indwelling catheters for Strong Moderate
UTIs long-term use due to a lower risk of UTIs. Routine use of antimicrobial coated
catheters is not recommended.
Catheter-related complications: For those using intermittent catheters, we suggest using single PVC (as Weak Low
UTIs opposed to multiple-use PVC), and special hydrophilic-coated catheters or pre-
lubricated catheters, as they may decrease the frequency of UTIs.
Catheter-related complications: We suggest using hydrophilic catheters, as they may cause less urethral Weak Moderate
Urethral trauma trauma.
Catheter-related complications: There is no evidence to suggest that the type of intermittent catheter impacts Weak Weak
Urethral trauma urethral stricture formation.
Patient perspective We recommend offering hydrophilic or pre-lubricated catheters to patients Strong Moderate
because of an improved bladder related QOL.
Economic analysis We suggest offering patients, if possible, HC catheters, as they are cost-effective Moderate Moderate
compared to single-use uncoated catheters due to the decreased incidence of
UTIs and increased QOL.
HC: hydrophilic-coated; IC: intermittent catheterization; QOL: quality of life; UTI: urinary tract infection.
material, cleaning method, and/or catheterization technique Duchesnay, Ferring, Medtronic, and Pfizer; has received grants/honoraria from Boston Scientific and
remain controversial topics in urology. Pfizer; and has participated in clinical trials supported by Astellas, Boston Scientific, and Medtronic.
The preferred IC strategy varies by settings and practices. Dr. Hickling has been an advisory board member for Pfizer; a speakers’ bureau member for Allergan,
There is no evidence that there is one best catheter for all Astellas, and Pfizer; has received grants/honoraria from Allergan, Astellas, and Pfizer; and has
patients. Selection of the ideal IC type/technique is a complex participated in clinical trials supported by Astellas. The remaining authors report no competing
personal or financial interests related to this work.
balance between patient’s motor functions, acceptance of the
procedure, QOL, and the economic implications. Whenever
possible, hydrophilic-coated or prelubricated catheters should Prior to publication, this guideline underwent review by the CUA Guidelines Committee, CUA members
be proposed to the patient as the first treatment option at large, the CUAJ Editorial Board, and the CUA Executive Board.
because they appear to lower the risk of UTI, may result in
less urethral trauma, and have higher convenience and ease
of use compared to conventional uncoated catheters.
Reuse of catheters may still be considered in specific References
clinical scenarios, however, the patient should be made
aware there are concerns regarding efficacy and that there 1. Weld KJ, Dmochowski RR. Effect of bladder management on urological complications in spinal cord injured
is limited evidence to support cleansing techniques for a patients. J Urol 2000;163:768-72. https://doi.org/10.1016/S0022-5347(05)67800-7
single-use product. Long-term cost-effectiveness of single- 2. Nicolle LE, Committee SL-TC. Urinary tract infections in long-term-care facilities. Infect Control Hosp
Epidemiol 2001;22:167-75. https://doi.org/10.1086/501886
use HC catheters was established from the perspective of 3. Peatfield R, Burt A, Smith P. Suprapubic catheterization after spinal cord injury: A followup report. Paraplegia
several international healthcare systems. 1983;21:220-6. https://doi.org/10.1038/sc.1983.35
Debate continues to linger regarding the best catheter 4. Kiddoo D, Sawatzky B, Bascu CD, et al. Randomized crossover trial of single use hydrophilic coated vs
material and/or technique in which upper urinary tract multiple use polyvinylchloride catheters for intermittent catheterization to determine incidence of urinary
infection. J Urol 2015;194:174-9. https://doi.org/10.1016/j.juro.2014.12.096
deterioration can be prevented while minimizing treatment- 5. Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am 1997;11:609-22.
related morbidity. Therefore, further high-quality RCTs are https://doi.org/10.1016/S0891-5520(05)70376-7
warranted. 6. Saint S, Lipsky BA. Preventing catheter-related bacteriuria: Should we? Can we? How? Arch Int Med
1999;159:800-8. https://doi.org/10.1001/archinte.159.8.800
Competing interests: Dr. Campeau has been an advisory board and speakers’ bureau member 7. Lapides J, Diokno AC, Silber SJ, et al. Clean, intermittent self-catheterization in the treatment of urinary
for Astellas and Pfizer; has received grants/honoraria from Allergan, Astellas, and Pfizer; and has tract disease. J Urol 2002;167:1131-3. https://doi.org/10.1016/S0022-5347(02)80359-7
participated in clinical trials supported by Pfizer. Dr. Baverstock has been a speaker for Allergan, 8. Cornejo-Dávila V, Durán-Ortiz S, Pacheco-Gahbler C. Incidence of urethral stricture in patients with
Astellas, BSCI, and Pfizer; and has participated in clinical trials supported by Astellas and Pfizer. Dr. spinal cord injury treated with clean intermittent self-catheterization. Urology 2017;99:260-4.
https://doi.org/10.1016/j.urology.2016.08.024
Carlson has been an advisory board member for Astellas and Pfizer; has received travel honoraria 9. Afsar S, Yemisci O, Cosar S, et al. Compliance with clean intermittent catheterization in spinal cord
from Allergan; and has participated in clinical trials supported by Astellas and Pfizer. Dr. Elterman injury patients: A long-term followup study. Spinal Cord 2013;51:645. https://doi.org/10.1038/
has been an advisory board and speakers’ bureau member for Allergan, Astellas, Boston Scientific, sc.2013.46
CUAJ • July 2020 • Volume 14, Issue 7 E287