Page 13 - CUA 2020_Endourology
P. 13
Unmoderated Posters 1: Prostate Cancer, Endourology, BPH
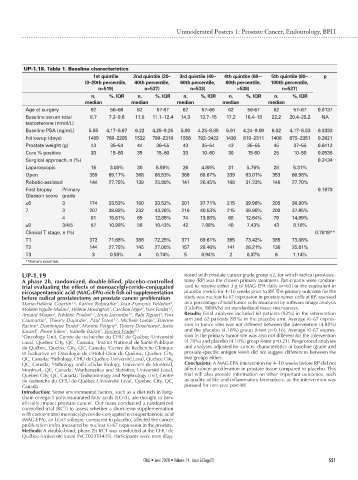
UP-1.18. Table 1. Baseline characteristics
1st quintile 2nd quintile (20– 3rd quintile (40– 4th quintile (60–- 5th quintile (80– p
(0–20th percentile, 40th percentile, 60th percentile, 80th percentile, 100th percentile,
n=519) n=537) n=533) =538) n=527)
n, %, IQR n, %, IQR n, %, IQR n, %, IQR n, %, IQR
median median median median median
Age at surgery 62 56–66 62 57-67 62 57–66 62 56-67 62 57–67 0.8137
Baseline serum total 8.7 7.3–9.6 11.8 11.1–12.4 14.3 13.7–15 17.2 16.4–18 22.2 20.4–25.2 NA
testosterone (nmol/L)
Baseline PSA (ng/mL) 5.85 4.17–8.67 6.22 4.25–9.25 5.90 4.25–8.85 5.91 4.24–9.09 6.02 4.17–8.53 0.3333
Followup (days) 1489 768–2205 1532 798–2318 1556 792–2422 1430 810–2311 1408 675–2351 0.2621
Prostate weight (g) 43 35–54 44 36–55 43 35–54 43 35–55 45 37–55 0.6412
Core % positive 33 15–60 35 15–60 33 10–60 30 15-60 25 10–50 0.0535
Surgical approach, n (%) 0.2434
Laparoscopic 16 3.08% 30 5.59% 26 4.88% 31 5.76% 28 5.31%
Open 359 69.17% 368 68.53% 366 68.67% 339 63.01% 353 66.98%
Robotic-assisted 144 27.75% 139 25.88% 141 26.45% 168 31.23% 146 27.70%
First biopsy Primary 0.1973
Gleason score grade
≤6 3 174 33.53% 180 33.52% 201 37.71% 215 39.96% 205 38.90%
7 3 207 39.88% 232 43.20% 216 40.53% 215 39.96% 200 37.95%
4 81 15.61% 69 12.85% 74 13.88% 68 12.64% 79 14.99%
≥8 3/4/5 57 10.98% 56 10.43% 42 7.88% 40 7.43% 43 8.16%
Clinical T stage, n (%) 0.7819**
T1 372 71.68% 388 72.25% 371 69.61% 395 73.42% 385 73.06%
T2 144 27.75% 145 27.00% 157 29.46% 141 26.21% 136 25.81%
T3 3 0.58% 4 0.74% 5 0.94% 2 0.37% 6 1.14%
**Fisher’s exact test.
UP-1.19 nosed with prostate cancer grade group ≥2, for which radical prostatec-
A phase 2b, randomized, double-blind, placebo-controlled tomy (RP) was the chosen primary treatment. Participants were random-
trial evaluating the effects of monoacylglyceride-conjugated ized to receive either 3 g of MAG-EPA daily (n=65) or the equivalent in
eicosapentaenoic acid (MAG-EPA)-rich fish oil supplementation placebo (n=65) for 4–10 weeks prior to RP. The primary outcome for the
before radical prostatectomy on prostate cancer proliferation study was nuclear Ki-67 expression in prostate tumor cells at RP, assessed
1
Marie-Hélène Guertin , Karine Robitaille , Jean-François Pelletier , as a percentage of total tumor cells measured by software image analysis
1,2
1
Molière Nguile-Makao , Hélène Hovington , Caroline Léger , Yves Fradet , (CaloPix, TRIBVN) on standardized tissue microarrays.
1,3
1
1
1
1,3
3
Arnaud Marien , Frédéric Pouliot , Louis Lacombe , Rabi Tiguert , Yves Results: Final analyses included 60 patients (92%) in the intervention
1,3
3
3
3
Caumartin , Thierry Dujardin , Paul Toren , Michele Lodde , Etienne arm and 62 patients (95%) in the placebo arm. Average Ki-67 expres-
3
1,3
5
4
6
4
Racine , Dominique Trudel , Martine Périgny , Thierry Duschesne , Josée sion in tumor sites was not different between the intervention (4.88%)
1
Savard , Pierre Julien , Isabelle Bairati , Vincent Fradet 1,3 and the placebo (4.18%) group (t-test p=0.16). Average Ki-67 expres-
1
7
1 Oncology Unit, Centre de recherche du CHU de Québec-Université sion in the primary tumor site was also not different for the intervention
2
Laval, Quebec City, QC, Canada; Institut National de Santé Publique (4.70%) and placebo (4.10%) group (t-test p=0.21). Per-protocol analyses
du Québec, Quebec City, QC, Canada; Centre de Recherche Clinique and analyses adjusted for cancer characteristics at baseline (grade and
3
et Évaluative en Oncologie de L’Hôtel-Dieu de Québec, Quebec City, prostate-specific antigen level) did not suggest differences between the
QC, Canada; Pathology, CHU de Québec-Université Laval, Quebec City, two groups either.
4
5
QC, Canada; Pathology and Cellular Biology, Université de Montréal, Conclusions: A MAG-EPA intervention for 4–10 weeks before RP did not
6
Montreal, QC, Canada; Mathematics and Statistics, Université Laval, affect cancer proliferation in prostate tissue compared to placebo. This
Quebec City, QC, Canada; Endocrinology and Nephrology Unit, Centre trial will also provide information on other important outcomes, such
7
de recherche du CHU de Québec-Université Laval, Quebec City, QC, as quality of life and inflammatory biomarkers, as the intervention was
Canada pursued for one-year post-RP.
Introduction: Some environmental factors, such as a diet rich in long-
chain omega-3 polyunsaturated fatty acids (LCn3), are thought to ben-
eficially impact prostate cancer. Our team conducted a randomized
controlled trial (RCT) to assess whether a short-term supplementation
with concentrated monoacylglyceride-conjugated eicosapentaenoic acid
(MAG-EPA), an LCn3 subtype, compared to placebo, affected the cancer
proliferation index measured by nuclear Ki-67 expression in the prostate.
Methods: A double-blind, phase 2b RCT was conducted at the CHU de
Québec–Université Laval (NCT02333435). Participants were men diag-
CUAJ • June 2020 • Volume 14, Issue 6(Suppl2) S51