Page 7 - CUA 2020_Onco_Testis-Kidney-Urothelial
P. 7
Moderated Posters 3: Uro-Oncology
Conclusions: Large renal masses (>4 cm) appear to have more aggres- taneously into the right flank. To quantify the metastatic burden of 786-0
sive cancer biology than their smaller counterparts (<4 cm), as reflected C14orf142 KO or scramble cells in distal organs, qPCR was performed
by a higher growth rate. However, metastatic rates and cancer-specific with primers specific for human Alu repeats. For statistical analysis, t-test
mortality are low in carefully selected patients on AS. was used to evaluate differences between groups with p≤0.05 accepted
as statistically significant.
MP-3.9 Results: The CRISPR-Cas 9 targeted genomic editing resulted in generation
of 786-0 clones with complete KO of C14orf142. Compared to 786-0
A novel nuclear protein as a therapeutic target to block metastasis scramble clones, C14orf142 KO clones demonstrated significant reduc-
of clear-cell renal cell carcinoma tion in extravasation on the avian embryo model (100% vs 46.33±8.37%,
Jan K. Rudzinski , Natasha Govindasamy , Konstantin Stoletov , Arun respectively, n=6, p≤0.05). Compared to NSG mice injected with 786-0
2
2
1,2
Raturi , John D Lewis 2 scramble clones, NSG mice implanted with 786-0 C14orf142 KO clones
2
1 Division of Urology, Department of Surgery, University of Alberta, demonstrated significant reduction in distant metastatic burden to lungs
Edmonton, AB, Canada; Department of Oncology, University of Alberta, (2∆∆CT 1.00+0.34 vs 0.08+0.01, respectively, n=10, p≤0.05), liver
2
Edmonton, AB, Canada (2∆∆CT 1.00+0.04 vs 0.52+0.14, respectively, n=10, p≤0.05), and brain
Support: Kidney Cancer Research Network and Kidney Cancer Canada (2∆∆CT 1.00+0.17 vs 0.27+0.06, respectively, n=10, p≤0.05).
Research Grant Conclusions: A novel nuclear protein, C14orf142, may play an important
Introduction: Whole human genome screen on human squamous cell role in cRCC vascular extravasation and distant metastasis in vivo.
carcinoma cells identified a panel of novel functional genes required for
metastatic dissemination in vivo. One such novel protein target encodes
for C14orf142, which is upregulated in metastatic clear-cell RCC (cRCC). MP-3.10
The objective was to characterize its impact on cRCC vascular extravasa- DDX41 expression induces worse prognosis and sensitivity to
tion and distant metastasis in vivo. nivolumab via sting signaling activation in clear-cell renal cell
Methods: cRCC cell lines, 786-0, were obtained from ATCC. Targeted carcinoma
genomic editing to knockout (KO) C14orf142 was achieved with CRISPR- Kenichiro Ikeda , Kohei Kobatake , Tetsutaro Hayashi , Jun Teishima , Akio
1
1
1
1
Cas 9 system. To study cancer cell vascular extravasation in vivo, 786-0 Matsubara 1
cells were injected intravenously into fertilized avian embryos. To deter- 1 Urology, Hiroshima University Hospital, Hiroshima, Japan
mine the impact of C14orf142 KO on in vivo spontaneous metastasis, a Introduction: Mutations of chromosome 3p, such as PBRM1, SETD2,
xenograft NSG nude mouse model was used. Six million 786-0 CRISPR and BAP1, have emerged as prognostic biomarker in clear-cell renal
KO or scramble clones were suspended in Matrigel and injected subcu- cell carcinoma (ccRCC). However, the response immune checkpoint
1,2
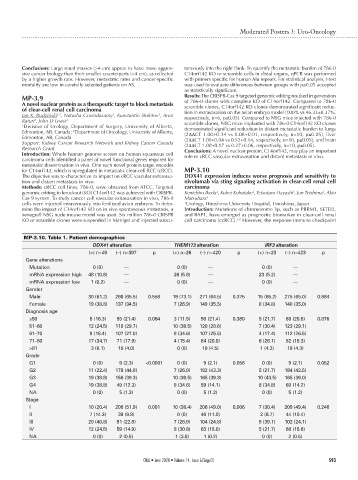
MP-3.10. Table 1. Patient demographics
DDX41 alteration THEM173 alteration IRF3 alteration
(+) n=49 (–) n=397 p (+) n=26 (–) n=420 p (+) n=23 (–) n=423 p
Gene alterations
Mutation 0 (0) — 0 (0) — 0 (0) —
mRNA expression high 48 (10.8) — 26 (5.8) — 23 (5.2) —
mRNA expression low 1 (0.2) — 0 (0) — 0 (0) —
Gender
Male 30 (61.2) 260 (65.5) 0.556 19 (73.1) 271 (64.5) 0.375 15 (65.2) 275 (65.0) 0.984
Female 19 (38.8) 137 (34.5) 7 (26.9) 149 (35.5) 8 (34.8) 148 (35.0)
Diagnosis age
≤50 8 (16.3) 85 (21.4) 0.064 3 (11.5) 90 (21.4) 0.380 5 (21.7) 88 (20.8) 0.876
51–60 12 (24.5) 118 (29.7) 10 (38.5) 120 (28.6) 7 (30.4) 123 (29.1)
61–70 9 (18.4) 107 (27.0) 9 (34.6) 107 (25.5) 4 (17.4) 112 (26.5)
71–80 17 (34.7) 71 (17.9) 4 (15.4) 84 (20.0) 6 (26.1) 82 (19.3)
>81 3 (6.1) 16 (4.0) 0 (0) 19 (4.5) 1 (4.3) 18 (4.3)
Grade
G1 0 (0) 9 (2.3) <0.0001 0 (0) 9 (2.1) 0.056 0 (0) 9 (2.1) 0.052
G2 11 (22.4) 178 (44.8) 7 (26.9) 182 (43.3) 5 (21.7) 184 (43.5)
G3 19 (38.8) 156 (39.3) 10 (38.5) 165 (39.3) 10 (43.5) 165 (39.0)
G4 19 (38.8) 49 (12.3) 9 (34.6) 59 (14.1) 8 (34.8) 60 (14.2)
NA 0 (0) 5 (1.3) 0 (0) 5 (1.2) 0 (0) 5 (1.2)
Stage
I 10 (20.4) 206 (51.9) 0.001 10 (38.4) 206 (49.0) 0.006 7 (30.4) 209 (49.4) 0.246
II 7 (14.3) 39 (9.8) 0 (0) 46 (11.0) 2 (8.7) 44 (10.4)
III 20 (40.8) 91 (22.9) 7 (26.9) 104 (24.8) 9 (39.1) 102 (24.1)
IV 12 (24.5) 59 (14.9) 8 (30.8) 63 (15.0) 5 (21.7) 66 (15.6)
NA 0 (0) 2 (0.5) 1 (3.8) 1 (0.2) 0 (0) 2 (0.5)
CUAJ • June 2020 • Volume 14, Issue 6(Suppl2) S93