Page 4 - CUA Adv Prostate Ca Drug Acccess Listing
P. 4
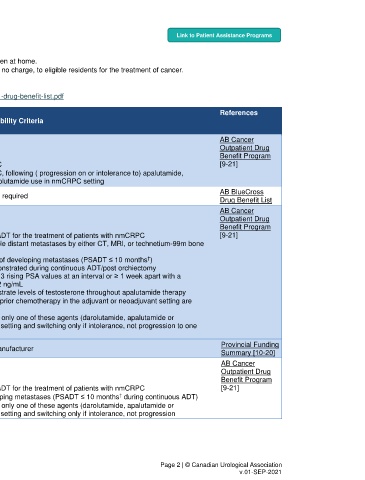
ALBERTA Link to Patient Assistance Programs
Funding:
Cancer patients may receive treatment at cancer centres, their community hospitals, or taken at home.
Alberta provides cancer drugs specified in the Outpatient Cancer Drug Benefit Program, at no charge, to eligible residents for the treatment of cancer.
Formulary:
Alberta Health Services https://www.albertahealthservices.ca/assets/programs/ps-1025651-drug-benefit-list.pdf
DRUG Strength, References
(Brand Name) Indication DIN†† Provincial Funding Eligibility Criteria
Manufacturer Route
Group 2* AB Cancer
Outpatient Drug
Abiraterone Eligibility: Benefit Program
(Zytiga) mCRPC Oral Multiple • Patients with mCRPC [9-21]
Janssen
• Patients with mCRPC, following ( progression on or intolerance to) apalutamide,
enzalutamide, or darolutamide use in nmCRPC setting
AB BlueCross
1
Alendronate Osteoporosis 70 mg PO Multiple Regular Benefit : No form required
Drug Benefit List
Group 2* AB Cancer
Outpatient Drug
Eligibility: Benefit Program
• In combination with ADT for the treatment of patients with nmCRPC [9-21]
o No detectable distant metastases by either CT, MRI, or technetium-99m bone
scan
†
o At high risk of developing metastases (PSADT ≤ 10 months )
Not o CRPC demonstrated during continuous ADT/post orchiectomy
Apalutamide nmCRPC Oral specified o Minimum of 3 rising PSA values at an interval or ≥ 1 week apart with a
(Erleada) last PSA > 2 ng/mL
Janssen o Maintain castrate levels of testosterone throughout apalutamide therapy
• Patients treated with prior chemotherapy in the adjuvant or neoadjuvant setting are
eligible
• Patients may receive only one of these agents (darolutamide, apalutamide or
enzalutamide) in this setting and switching only if intolerance, not progression to one
agent
Not Provincial Funding
mCSPC - Under negotiation with manufacturer
specified Summary [10-20]
Group 2* AB Cancer
Outpatient Drug
Darolutamide Not Eligibility: Benefit Program
(Nubeqa) nmCRPC Oral specified • In combination with ADT for the treatment of patients with nmCRPC [9-21]
Bayer • At high risk of developing metastases (PSADT ≤ 10 months during continuous ADT)
†
• Patients may receive only one of these agents (darolutamide, apalutamide or
enzalutamide) in this setting and switching only if intolerance, not progression
Page 2 | © Canadian Urological Association
v.01-SEP-2021