Page 7 - CUA Adv Prostate Ca Drug Acccess Listing
P. 7
BRITISH COLUMBIA Link to Patient Assistance Programs
Funding:
Medications for active cancer treatment are funded by BC Cancer for all BC Medical Services Plan patients (including First Nations Health Authority clients). These medications are
supplied at no charge to registered BC cancer patients at BC Cancer Centres and Clinics.
Patients may also have private drug plans that cover some or all of medication costs.
Formulary:
British Columbia Cancer Agency Benefit Drug List
http://www.bccancer.bc.ca/systemic-therapy-site/Documents/Policy%20and%20Forms/Benefit%20Drug%20List.pdf
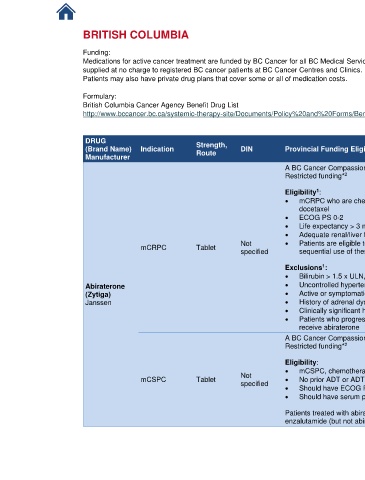
DRUG Strength, References
(Brand Name) Indication Route DIN Provincial Funding Eligibility Criteria
Manufacturer
A BC Cancer Compassionate Access Program request must be approved prior to treatment. 1. BC Cancer
1
Restricted funding* Protocol
2
UGUPABI [8-
Eligibility : 21]
1
• mCRPC who are chemotherapy naïve or have received prior chemotherapy containing 2. BC Cancer
docetaxel Benefit Drug
• ECOG PS 0-2 List [9-21]
• Life expectancy > 3 months
• Adequate renal/liver function and serum potassium levels
mCRPC Tablet Not • Patients are eligible to received abiraterone OR enzalutamide OR apalutamide but not
specified sequential use of these agents
1
Exclusions :
• Bilirubin > 1.5 x ULN, AST or ALT > 2.5 x ULN
Abiraterone • Uncontrolled hypertension (systolic BP > 160 mmHg or diastolic BP > 95 mmHg)
(Zytiga) • Active or symptomatic viral hepatitis or chronic liver disease
Janssen • History of adrenal dysfunction
• Clinically significant heart disease (LVEF < 50% at baseline)
• Patients who progressed on apalutamide or enzalutamide in nmCRPC are not eligible to
receive abiraterone
1
A BC Cancer Compassionate Access Program request must be approved prior to treatment. 1. BC Cancer
2
Restricted funding* Protocol
UGUMCSPAB
Eligibility: I [9-21]
• mCSPC, chemotherapy naïve or received prior chemotherapy containing docetaxel 2. BC Cancer
Not
mCSPC Tablet • No prior ADT or ADT for not > 6 months for mCSPC Benefit Drug
specified
• Should have ECOG PS 0-2 List [9-21]
• Should have serum potassium > 3.5 mmol/L
Patients treated with abiraterone for mCSPC and develop mCRPC are eligible to receive
enzalutamide (but not abiraterone),
Page 4 | © Canadian Urological Association
v.01-SEP-2021