Page 10 - Guideline
P. 10
09010:Layout 1 3/18/10 9:26 PM Page E28
Wood et al.
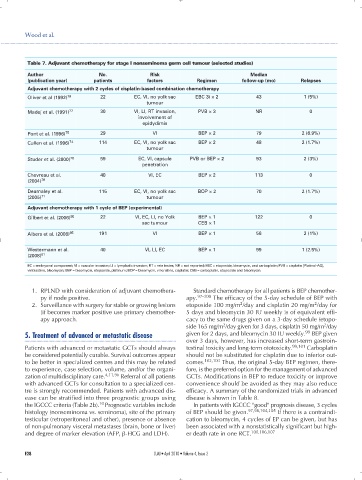
Table 7. Adjuvant chemotherapy for stage I nonseminoma germ cell tumour (selected studies)
Author No. Risk Median
(publication year) patients factors Regimen follow-up (mo) Relapses
Adjuvant chemotherapy with 2 cycles of cisplatin-based combination chemotherapy
Oliver et al (1992) 76 22 EC, VI, no yolk sac EBC 3i × 2 43 1 (5%)
tumour
Madej et al. (1991) 77 30 VI, LI, RT invasion, PVB × 3 NR 0
involvement of
epidydimis
Pont et al. (1996) 75 29 VI BEP × 2 79 2 (6.9%)
Cullen et al. (1996) 74 114 EC, VI, no yolk sac BEP × 2 48 2 (1.7%)
tumour
Studer et al. (2000) 78 59 EC, VI, capsule PVB or BEP × 2 93 2 (3%)
penetration
Chevreau et al. 40 VI, EC BEP × 2 113 0
(2004) 79
Dearnaley et al. 115 EC, VI, no yolk sac BOP × 2 70 2 (1.7%)
(2005) 71 tumour
Adjuvant chemotherapy with 1 cycle of BEP (experimental)
Gilbert et al. (2006) 80 22 VI, EC, LI, no Yolk BEP × 1 122 0
sac tumour CEB × 1
Albers et al. (2008) 65 191 VI BEP × 1 56 2 (1%)
Westermann et al. 40 VI, LI, EC BEP × 1 99 1 (2.5%)
(2008) 81
EC = embryonal component; VI = vascular invasion; LI = lymphatic invasion; RT = rete testes; NR = not reported; EBC = etoposide, bleomycin, and carboplatin; PVB = cisplatin [Platinol-AQ],
vinblastine, bleomycin; BEP = bleomycin, etoposide, platinum; BOP = bleomycin, vincristine, cisplatin; CEB = carboplatin, etoposide and bleomycin.
1. RPLND with consideration of adjuvant chemothera- Standard chemotherapy for all patients is BEP chemother-
py if node positive. apy. 97-100 The efficacy of the 5-day schedule of BEP with
2
2
2. Surveillance with surgery for stable or growing lesions etoposide 100 mg/m /day and cisplatin 20 mg/m /day for
(if becomes marker positive use primary chemother- 5 days and bleomycin 30 IU weekly is of equivalent effi-
apy approach. cacy to the same drugs given on a 3-day schedule (etopo-
2
2
side 165 mg/m /day given for 3 days, cisplatin 50 mg/m /day
5. Treatment of advanced or metastatic disease given for 2 days, and bleomycin 30 IU weekly. 99 BEP given
over 3 days, however, has increased short-term gastroin-
Patients with advanced or metastatic GCTs should always testinal toxicity and long-term ototoxicity. 99,101 Carboplatin
be considered potentially curable. Survival outcomes appear should not be substituted for cisplatin due to inferior out-
to be better in specialized centres and this may be related comes. 102,103 Thus, the original 5-day BEP regimen, there-
to experience, case selection, volume, and/or the organi- fore, is the preferred option for the management of advanced
zation of multidisciplinary care. 4,11,96 Referral of all patients GCTs. Modifications in BEP to reduce toxicity or improve
with advanced GCTs for consultation to a specialized cen- convenience should be avoided as they may also reduce
tre is strongly recommended. Patients with advanced dis- efficacy. A summary of the randomized trials in advanced
ease can be stratified into three prognostic groups using disease is shown in Table 8.
10
the IGCCC criteria (Table 2b). Prognostic variables include In patients with IGCCC “good” prognosis disease, 3 cycles
histology (nonseminoma vs. seminoma), site of the primary of BEP should be given. 97,98,104,105 If there is a contraindi-
testicular (retroperitoneal and other), presence or absence cation to bleomycin, 4 cycles of EP can be given, but has
of non-pulmonary visceral metastases (brain, bone or liver) been associated with a nonstatistically significant but high-
and degree of marker elevation (AFP, β-HCG and LDH). er death rate in one RCT. 100,106,107
E28 CUAJ • April 2010 • Volume 4, Issue 2